Competitive inhibition of natural antisense Sok-RNA interactions activates Hok-mediated cell killing in Escherichia coli - PubMed (original) (raw)
Competitive inhibition of natural antisense Sok-RNA interactions activates Hok-mediated cell killing in Escherichia coli
Omid R Faridani et al. Nucleic Acids Res. 2006.
Abstract
Short regulatory RNAs are widespread in bacteria, and many function through antisense recognition of mRNA. Among the best studied antisense transcripts are RNA antitoxins that repress toxin mRNA translation. The hok/sok locus of plasmid R1 from Escherichia coli is an established model for RNA antitoxin action. Base-pairing between hok mRNA and Sok-antisense-RNA increases plasmid maintenance through post-segregational-killing of plasmid-free progeny cells. To test the model and the idea that sequestration of Sok-RNA activity could provide a novel antimicrobial strategy, we designed anti Sok peptide nucleic acid (PNA) oligomers that, according to the model, would act as competitive inhibitors of hok mRNA::Sok-RNA interactions. In hok/sok-carrying cells, anti Sok PNAs were more bactericidal than rifampicin. Also, anti Sok PNAs induced ghost cell morphology and an accumulation of mature hok mRNA, consistent with cell killing through synthesis of Hok protein. The results support the sense/antisense model for hok mRNA repression by Sok-RNA and demonstrate that antisense agents can be used to out-compete RNA::RNA interactions in bacteria. Finally, BLAST analyses of approximately 200 prokaryotic genomes revealed that many enteric bacteria have multiple hok/sok homologous and analogous RNA-regulated toxin-antitoxin loci. Therefore, it is possible to activate suicide in bacteria by targeting antitoxins.
Figures
Figure 1
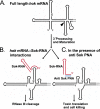
Schematic model of the hok/sok TA system and the Sok-RNA inhibition strategy used in this study. (A) Full-length hok mRNA folds into a compact form in which the 5′ and 3′ ends of the molecule pair. The ends of the molecule make an exact match, thus generating a highly folded and ‘blunt-ended’ RNA structure. The sequestering of the mRNA 3′ end reduces the rate of 3′-processing by polynucleotide phosphorylase and ribonuclease II (15). However, the 3′ exoenzymes removes the terminal 39 nt at the 3′ end of hok mRNA at a low rate. The arrow-head points to the bottom of the stem–loop structure, which acts as a ‘road-block’ for the 3′ end trimming. (B) Via its 5′ end single-stranded tail, Sok-RNA (shown in red) recognises a single-stranded stem–loop present only in the truncated, refolded hok mRNA. This is because the 3′ trimming of full-length hok mRNA releases the very 5′ end of the mRNA and this release triggers a major refolding of the mRNA 5′ end that results in the formation of the antisense RNA binding stem–loop structure (26,27). The refolded isoform of the mRNA is metabolically very stable and binds Sok-RNA avidly, but can also be bound by ribosomes and therefore be translated (26). In the presence of excess Sok-RNA rapid binding of the antisense RNA prevents ribosome binding to hok mRNA and thus prevents its translation (12,26). Eventually, the hok mRNA::Sok-RNA duplex is formed and then rapidly cleaved (scavenged) by RNase III (18). Therefore, the truncated form of hok mRNA does not accumulate in Sok-RNA containing cells. The RNase III cleavage of the hok mRNA::Sok-RNA duplex is not required for inhibition of hok mRNA translation (41). (C) Sok-RNA inhibition strategy. PNAs complementary to the single-stranded 5′ end of Sok-RNA (shown in blue) prevent Sok-RNA binding to hok mRNA and thereby induce hok translation, synthesis of the lethal Hok protein and cell killing.
Figure 2
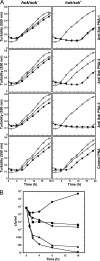
Effects of anti Sok PNAs on the growth of E. coli carrying hok/sok system. (A) Turbidity in an untreated culture (open circle) and cultures including anti Sok PNAs added at 8 μM (open triangle) and 10 μM (closed square). (B) C.f.u. in an untreated culture (closed circle) and cultures containing anti Sok PNA 5 μM (closed triangle) and 10 μM (closed inverted triangle) and rifampicin at 100 μM = 122 μg/ml (closed square) and 200 μM = 244 μg/ml (closed diamond).
Figure 3
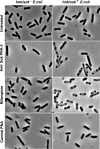
Effect of anti Sok PNAs on cell morphology of E.coli carrying hok/sok. PNAs and rifampicin were added to growing cells carrying control or _hok/sok_-containing plasmids.
Figure 4
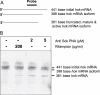
Effect of anti Sok PNA treatment on hok mRNA processing. (A) hok mRNA isoforms. The 441 and 398 nt long RNAs are initial hok mRNAs that are processed into the mature, truncated isoform of 361 bp hok mRNA (15,19). In the presence of an excess of active Sok-RNA, the truncated version of the hok mRNA does not accumulate due to rapid Sok-RNA binding followed by rapid RNase III processing of the hok mRNA::Sok-RNA duplex. The long isoforms of hok mRNA bind Sok-RNA much slower and therefore accumulate even in the presence of Sok-RNA (26). The long versions of hok mRNA cannot bind to ribosomes and be translated and are not harmful to the cell. (B) Northern analysis of hok mRNAs following exposure to anti Sok PNA or rifampicin. The initial transcripts and 3′ end truncated, mature and active isoforms of hok mRNA are indicated.
Figure 5
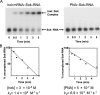
PNA::Sok-RNA complex formation in vitro and binding kinetics. (A) 32P-Radiolabeled Sok-RNA incubated with a 15-fold molar excess of unlabelled anti Sok PNA (500 nM) or hok mRNA (30 nM), fractionated in a polyacrylamide/urea/TBE gel and analysed by autoradiography and densitometry. (B) Plots of the fractions of uncomplexed Sok-RNA as a function of time of incubation, with the binding-rate constants indicated.
Figure 6
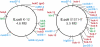
Localization of hok/sok (red) and the hok/_sok_-like ldr loci (green) on two E.coli chromosomes. Protein-regulated TA loci (blue) were included for comparison (34). The genes were found using tblastn (Supplementary Table S1) and annotated with VectorNTI.
Similar articles
- The hok mRNA family.
Steif A, Meyer IM. Steif A, et al. RNA Biol. 2012 Dec;9(12):1399-404. doi: 10.4161/rna.22746. Epub 2012 Dec 1. RNA Biol. 2012. PMID: 23324554 - RNA antitoxins.
Gerdes K, Wagner EG. Gerdes K, et al. Curr Opin Microbiol. 2007 Apr;10(2):117-24. doi: 10.1016/j.mib.2007.03.003. Epub 2007 Mar 21. Curr Opin Microbiol. 2007. PMID: 17376733 Review. - The hok killer gene family in gram-negative bacteria.
Gerdes K, Poulsen LK, Thisted T, Nielsen AK, Martinussen J, Andreasen PH. Gerdes K, et al. New Biol. 1990 Nov;2(11):946-56. New Biol. 1990. PMID: 2101633 Review.
Cited by
- Bacterial Toxin-Antitoxin Systems' Cross-Interactions-Implications for Practical Use in Medicine and Biotechnology.
Boss L, Kędzierska B. Boss L, et al. Toxins (Basel). 2023 Jun 4;15(6):380. doi: 10.3390/toxins15060380. Toxins (Basel). 2023. PMID: 37368681 Free PMC article. Review. - A SARS-CoV-2 oral vaccine development strategy based on the attenuated Salmonella type III secretion system.
Wu L, Li L, Yin X, Li C, Xin W, Liu L, Hua Z. Wu L, et al. J Appl Microbiol. 2022 Oct;133(4):2484-2500. doi: 10.1111/jam.15720. Epub 2022 Jul 31. J Appl Microbiol. 2022. PMID: 35858677 Free PMC article. - Phage Genes Induce Quorum Sensing Signal Release through Membrane Vesicle Formation.
Yasuda M, Yamamoto T, Nagakubo T, Morinaga K, Obana N, Nomura N, Toyofuku M. Yasuda M, et al. Microbes Environ. 2022;37(1):ME21067. doi: 10.1264/jsme2.ME21067. Microbes Environ. 2022. PMID: 35082176 Free PMC article. - Activation of metabolic and stress responses during subtoxic expression of the type I toxin hok in Erwinia amylovora.
Peng J, Triplett LR, Sundin GW. Peng J, et al. BMC Genomics. 2021 Jan 22;22(1):74. doi: 10.1186/s12864-021-07376-w. BMC Genomics. 2021. PMID: 33482720 Free PMC article. - Doxycycline induces Hok toxin killing in host E. coli.
Chukwudi CU, Good L. Chukwudi CU, et al. PLoS One. 2020 Jul 6;15(7):e0235633. doi: 10.1371/journal.pone.0235633. eCollection 2020. PLoS One. 2020. PMID: 32628709 Free PMC article.
References
- Szymanski M., Barciszewska M.Z., Zywicki M., Barciszewski J. Noncoding RNA transcripts. J. Appl. Genet. 2003;44:1–19. - PubMed
- Storz G., Altuvia S., Wassarman K.M. An abundance of RNA regulators. Annu. Rev. Biochem. 2005;74:199–217. - PubMed
- Wagner E.G., Altuvia S., Romby P. Antisense RNAs in bacteria and their genetic elements. Adv. Genet. 2002;46:361–398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials