Flagellin suppresses epithelial apoptosis and limits disease during enteric infection - PubMed (original) (raw)
Flagellin suppresses epithelial apoptosis and limits disease during enteric infection
Matam Vijay-Kumar et al. Am J Pathol. 2006 Nov.
Abstract
Flagellin, the primary component of bacterial flagella, is a potent activator of toll-like receptor 5 (TLR5) signaling and is a major proinflammatory determinant of enteropathogenic Salmonella. In accordance with this, we report here that aflagellate Salmonella mutants are impaired in their ability to up-regulate proinflammatory and anti-apoptotic effector molecules in murine models of salmonellosis and that these mutants elicit markedly reduced early mucosal inflammation relative to their isogenic parent strains. Conversely, aflagellate bacteria were more potent activators of epithelial caspases and subsequent apoptosis. These phenomena correlated with a delayed but markedly exacerbated mucosal inflammation at the later stages of infection as well as elevated extra-intestinal and systemic bacterial load, culminating in a more severe clinical outcome. Systemic administration of exogenous flagellin primarily reversed the deleterious effects of in vivo Salmonella infection. These observations indicate that in Salmonella infection, flagellin plays a dominant role in activation of not only innate immunity but also anti-apoptotic processes in epithelial cells. These latter TLR-mediated responses that delay epithelial apoptosis may be as critical to mucosal defense as the classic acute inflammatory response. This notion is consistent with the emerging paradigm that specific TLR ligands may have a fundamental cytoprotective effect during inflammatory stress.
Figures
Figure 1
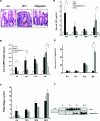
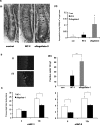
Aflagellate S. typhimurium exhibits enhanced but delayed inflammation in a murine model of enteric Salmonellosis. BALB/cj mice were pretreated with streptomycin and infected as indicated with Salmonella (WT-1, aflagellate-1, and FliD) and sacrificed at 6, 12, 24, and 48 hours after infection as described in Materials and Methods. A: H&E-stained cecal mucosal images (12 hours after infection) of control (streptomycin alone), WT-1, and aflagellate-1. Arrow denotes neutrophil influx. B: Cecal MPO activity at different time points. C: Quantification of intestinal inflammation. Sections were graded on a semiquantitative scale as described in Materials and Methods. Data are aggregate of five mice for each time point. D and E: Enzyme-linked immunosorbent assay for serum KC (D) and IL-6 (E). F: Representative immunoblot for serum lipocalin-2/NGAL from three mice in each group. *P < 0.05; **P < 0.01. Original magnifications, ×40.
Figure 2
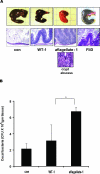
Cecal pathology of Salmonella infection after streptomycin pretreated. A: Top: gross cecal appearance; bottom: H&E-stained cecal images (48 hours after infection) with indicated strain. Inset: Crypt abscess in aflagellate-1-infected mice cecum. B: Adherent and invaded cecal bacterial load (Salmonella) at 48 hours after infection. *P < 0.05. Original magnifications, ×10; ×40 (inset).
Figure 3
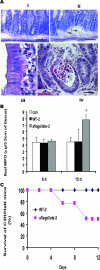
Aflagellate S. enteritidis causes a hypervirulent phenotype in model systemic murine infection. A: Inflammatory responses in C3H/HeN mice after exposure to S. enteritidis (WT-2 or aflagellate-2). H&E-stained frozen sections. i: Ileum, 6 days after infection showing normal crypt histology. ii–iv: Ileum, 12 days after infection showing edema (arrows) (ii) and cellular infiltrate between crypts (iii) and (iv) jejunum, 12 days after infec-tion showing the presence of neutrophils (arrows) in the lamina propria (LP) and transepithelial migration of PMNs into crypt abscesses. B: Ileal MPO activity. Aflagellate-2-infected versus control, or WT-2, *P < 0.05. C: Survival (percent) of C3H/HeN mice after oral infection with 108 CFU with WT-2 or aflagellate-2 as indicated. Scale bars = 10 μm (i, ii); 10 μm (iii); 25 μm (iv).
Figure 4
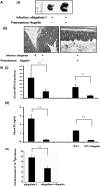
Aflagellate Salmonella invades more efficiently than WT. A: Counts of GFP-labeled invaded bacteria. B: Localization of GFP-labeled Salmonella enteritidis (aflagellate-2) in mouse ileum 4 hours after inoculation of an isolated ligated loop with 109 bacteria. Ligated loops were performed 8 days after infection with WT-2 or aflagellate-2. Representative confocal images of resin-embedded tissue sections showing the presence of bacteria within epithelial cells and LP of upper villus (i), mid villus (ii), and crypt (iii) regions. Intestinal membranes labeled with Datura stromonium lectin (DSL) and nuclei labeled with DAPI. *P < 0.05. Scale bars = 10 μm.
Figure 5
Exogenously administered flagellin can reverse tissue injury caused by aflagellate Salmonella. BALB/cj mice infected as described in Materials and Methods. A: Gross (i) and histological (ii) details (H&E stained) of the ceca at 48 hours after infection either with WT-1 or aflagellate-1 alone or with flagellin pretreatment. Gross cecal pathology was not different in WT-1 infection with or without flagellin pretreatment. B: Cecal MPO (i), serum KC (ii), and cecal bacteria (iii) counted on selective McConkey agar plates (tetracycline, 13 μg/ml), 48 hours after infection without or with flagellin (20 μg/mouse, i.p.) treatment 2 hours before infection with aflagellate-1. **P < 0.01.
Figure 6
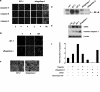
Aflagellate Salmonella elicit increased apoptosis in a murine model of infection. A: C3H/HeN-S. enterica system. i: Representative H&E images showing apoptotic bodies (arrows) in the lower villus/crypt regions of ileum in control C3H/HeN mice or animals infected with WT-2 or aflagellate-2, 12 days after infection. ii: Quantification of apoptosis 12 days after infection. B: BALB/c-S. typhimurium system. Caspase-3 staining in mouse ceca 12 hours after infection with WT-1 or aflagellate-1. Data are aggregate of five mice infected with the indicated strain. i: Control, ii: aflagellate-1. iii: Quantification of positive cells from (10 hpf) in BALB/cj mice cecum. C: qRT-PCR of cIAP-1 and cIAP-2 transcript in ceca infected with WT-1 or aflagellate-1. Data are aggregate of five mice infected with the indicated strain. qRT-PCR reactions were performed in triplicate for each sample and normalized to 18s ribosomal RNA. The relative levels of RNA were determined by comparing with the uninfected group *P < 0.05, **P < 0.01. Scale bars = 10 μm.
Figure 7
Aflagellate Salmonella are more potent inducers of caspases and apoptosis in vitro. A: Fluorescent microscopy of IEC-6 cells showing active caspase -8, -9, and -3 staining, after infection with WT-1 or aflagellate-1 for 2, 4, and 8 hours. B: TUNEL staining of IEC-6 cells 4, 8, and 12 hours after infection with WT-1 or aflagellate-1. C: Immunofluorescence detection of RelA in Caco-2 cells exposed to either S. enteritidis WT-2 or aflagellate-2 for 2 hours. D: Electrophoretic mobility shift assay for NF-κB activation in Caco-2 cells exposed to indicated bacteria for 2 hours. E: T84 cells were treated basolaterally for 8 hours with flagellin (100 ng/ml) or apically with WT-1 or aflagellate-1 (multiplicity of infection 30). Cell lysates were subjected to immunoblot with indicated antibodies, and β-actin was used as a loading control. F: In vitro cytoprotective effect of flagellin. IEC-6 cells were pretreated with flagellin (10 ng/ml) for 4 hours and subsequently infected with aflagellate-1 for 6 hours. Caspase-3 activation was quantified in cell lysates using luminescent substrate Caspase-Glo 3/7 assay (Promega). zVAD-FMK and staurosporine were included as positive controls for blocking or activating apoptosis, respectively. Data are representative of five experimental repeats.
Figure 8
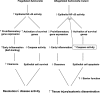
Diagram, see Discussion.
Similar articles
- Flagellin acting via TLR5 is the major activator of key signaling pathways leading to NF-kappa B and proinflammatory gene program activation in intestinal epithelial cells.
Tallant T, Deb A, Kar N, Lupica J, de Veer MJ, DiDonato JA. Tallant T, et al. BMC Microbiol. 2004 Aug 23;4:33. doi: 10.1186/1471-2180-4-33. BMC Microbiol. 2004. PMID: 15324458 Free PMC article. - Flagellin/TLR5 responses in epithelia reveal intertwined activation of inflammatory and apoptotic pathways.
Zeng H, Wu H, Sloane V, Jones R, Yu Y, Lin P, Gewirtz AT, Neish AS. Zeng H, et al. Am J Physiol Gastrointest Liver Physiol. 2006 Jan;290(1):G96-G108. doi: 10.1152/ajpgi.00273.2005. Epub 2005 Sep 22. Am J Physiol Gastrointest Liver Physiol. 2006. PMID: 16179598 Free PMC article. - Flagellin, a novel mediator of Salmonella-induced epithelial activation and systemic inflammation: I kappa B alpha degradation, induction of nitric oxide synthase, induction of proinflammatory mediators, and cardiovascular dysfunction.
Eaves-Pyles T, Murthy K, Liaudet L, Virág L, Ross G, Soriano FG, Szabó C, Salzman AL. Eaves-Pyles T, et al. J Immunol. 2001 Jan 15;166(2):1248-60. doi: 10.4049/jimmunol.166.2.1248. J Immunol. 2001. PMID: 11145708 - Flag in the crossroads: flagellin modulates innate and adaptive immunity.
Gewirtz AT. Gewirtz AT. Curr Opin Gastroenterol. 2006 Jan;22(1):8-12. doi: 10.1097/01.mog.0000194791.59337.28. Curr Opin Gastroenterol. 2006. PMID: 16319670 Review. - Salmonella flagellin, a microbial target of the innate and adaptive immune system.
Salazar-Gonzalez RM, McSorley SJ. Salazar-Gonzalez RM, et al. Immunol Lett. 2005 Nov 15;101(2):117-22. doi: 10.1016/j.imlet.2005.05.004. Epub 2005 Jun 6. Immunol Lett. 2005. PMID: 15975666 Review.
Cited by
- Salmonella enterica serovar typhimurium invades fibroblasts by multiple routes differing from the entry into epithelial cells.
Aiastui A, Pucciarelli MG, García-del Portillo F. Aiastui A, et al. Infect Immun. 2010 Jun;78(6):2700-13. doi: 10.1128/IAI.01389-09. Epub 2010 Apr 5. Infect Immun. 2010. PMID: 20368348 Free PMC article. - Activation of motility by sensing short-chain fatty acids via two steps in a flagellar gene regulatory cascade in enterohemorrhagic Escherichia coli.
Tobe T, Nakanishi N, Sugimoto N. Tobe T, et al. Infect Immun. 2011 Mar;79(3):1016-24. doi: 10.1128/IAI.00927-10. Epub 2010 Dec 13. Infect Immun. 2011. PMID: 21149585 Free PMC article. - Effects in the use of a genetically engineered strain of Lactococcus lactis delivering in situ IL-10 as a therapy to treat low-grade colon inflammation.
Martín R, Chain F, Miquel S, Natividad JM, Sokol H, Verdu EF, Langella P, Bermúdez-Humarán LG. Martín R, et al. Hum Vaccin Immunother. 2014;10(6):1611-21. doi: 10.4161/hv.28549. Epub 2014 Apr 14. Hum Vaccin Immunother. 2014. PMID: 24732667 Free PMC article. - Immunization with Bivalent Flagellin Protects Mice against Fatal Pseudomonas aeruginosa Pneumonia.
Behrouz B, Hashemi FB, Fatemi MJ, Naghavi S, Irajian G, Halabian R, Imani Fooladi AA. Behrouz B, et al. J Immunol Res. 2017;2017:5689709. doi: 10.1155/2017/5689709. Epub 2017 Oct 19. J Immunol Res. 2017. PMID: 29201922 Free PMC article. - Interplay between enterobactin, myeloperoxidase and lipocalin 2 regulates E. coli survival in the inflamed gut.
Singh V, Yeoh BS, Xiao X, Kumar M, Bachman M, Borregaard N, Joe B, Vijay-Kumar M. Singh V, et al. Nat Commun. 2015 May 12;6:7113. doi: 10.1038/ncomms8113. Nat Commun. 2015. PMID: 25964185 Free PMC article.
References
- Turner JR. Functional morphology of the intestinal mucosae: from crypts to tips. Hecht G, editor. Washington, DC: ASM Press,; Microbial Pathogenesis and the Intestinal Epithelial Cell. 2003:pp 1–22.
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. - PubMed
- Barton GM, Medzhitov R. Toll-like receptors and their ligands. Curr Top Microbiol Immunol. 2002;270:81–92. - PubMed
- O’Neill LAJ. Signal transduction pathways activated by the IL-1 receptor/Toll-like receptor superfamily. Beutler B, Wagner H, editors. New York: Springer,; Toll-Like Receptor Family Members and Their Ligands. 2003:pp 47–62.
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK061417/DK/NIDDK NIH HHS/United States
- DK061417/DK/NIDDK NIH HHS/United States
- R01 AI51282/AI/NIAID NIH HHS/United States
- DK071604/DK/NIDDK NIH HHS/United States
- R01 AI051282/AI/NIAID NIH HHS/United States
- R24 DK064399/DK/NIDDK NIH HHS/United States
- R01 DK071604/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical