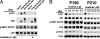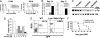Targeting multiple kinase pathways in leukemic progenitors and stem cells is essential for improved treatment of Ph+ leukemia in mice - PubMed (original) (raw)
Targeting multiple kinase pathways in leukemic progenitors and stem cells is essential for improved treatment of Ph+ leukemia in mice
Yiguo Hu et al. Proc Natl Acad Sci U S A. 2006.
Abstract
It is generally believed that shutting down the kinase activity of BCR-ABL by imatinib will completely inhibit its functions, leading to inactivation of its downstream signaling pathways and cure of the disease. Imatinib is highly effective at treating human Philadelphia chromosome-positive (Ph(+)) chronic myeloid leukemia (CML) in chronic phase but not Ph(+) B cell acute lymphoblastic leukemia (B-ALL) and CML blast crisis. We find that SRC kinases activated by BCR-ABL remain fully active in imatinib-treated mouse leukemic cells, suggesting that imatinib does not inactivate all BCR-ABL-activated signaling pathways. This SRC pathway is essential for leukemic cells to survive imatinib treatment and for CML transition to lymphoid blast crisis. Inhibition of both SRC and BCR-ABL kinase activities by dasatinib affords complete B-ALL remission. However, curing B-ALL and CML mice requires killing leukemic stem cells insensitive to both imatinib and dasatinib. Besides BCR-ABL and SRC kinases, stem cell pathways must be targeted for curative therapy of Ph(+) leukemia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
SRC kinases remain active after inhibition of BCR-ABL kinase activity by imatinib. (A) Activation of SRC kinases by BCR-ABL does not require BCR-ABL kinase activity. P190- or P210-expressing ENU cells were cultured in the presence or absence of imatinib for 12 h, and ENU cells bearing empty vector were used as controls. Protein lysates were analyzed by Western blotting with antibodies against phosphotyrosine (_p_-Tyr), ABL, activated SRC kinases (_p_-SRC-Tyr-416) (29), and LYN. (B) BCR-ABL-transduced BM cells were cultured under Whitlock–Witte conditions for 5 days. The cells were treated with imatinib at the concentrations indicated for 2 days. Protein lysates were analyzed by Western blotting with the antibodies indicated.
Fig. 2.
SRC kinases play a critical role in maintaining survival and promoting proliferation of pre-B leukemic cells. (A) BM cells from B6 mice were transduced with the empty vector or v-SRC retrovirus and cultured under Whitlock–Witte conditions for 14 days. (B) Dasatinib inhibits activity of both BCR-ABL and SRC kinases. BCR-ABL-transduced BM cells were cultured under Whitlock–Witte conditions for 5 days. Different concentrations of dasatinib were added to the culture for 48 h, and protein lysates were analyzed by Western blotting. (C) Inhibition of SRC kinases reduces survival of BCR-ABL-T315I-expressing B-lymphoid cells. BCR-ABL-T315I-transduced BM cells were cultured at 1 × 105 cells per well in 24-well plates, and different concentrations of dasatinib were added to the culture for 5 or 7 days. Viable cells were counted. (D) Therapeutic effect of imatinib and dasatinib on BCR-ABL-T315I-induced B-ALL. BMT, BM transplantation. (E) In vivo inhibition of SRC kinase activity with dasatinib. Mice with BCR-ABL-T315I-induced B-ALL were treated with a placebo or dasatinib for 3 days. After the last dose, leukemic cells from peripheral blood of the mice were analyzed by Western blotting. Each lane represents a mouse from the indicated treatment group. (F) BCR-ABL-transduced wild-type or Lyn/Hck/Fgr triple knockout BM cells were transplanted into wild-type recipient mice to induce B-ALL. GFP+ cell counts (percentage of GFP+ cells × white blood cell count) were measured at different time points after the induction of leukemia. (G) Percentages of GFP+ B-leukemic cells in peripheral blood were determined by FACS analysis as described in F.
Fig. 3.
Simultaneous targeting of kinase activity of both BCR-ABL and SRC kinases results in long-term survival of mice with B-ALL. (A) Mice with BCR-ABL-induced B-ALL were treated with a placebo, imatinib, or dasatinib. BMT, BM transplantation. (B) Reduction of GFP+ leukemic cells in peripheral blood of the treated B-ALL mice. (C) In vivo inhibition of BCR-ABL autophosphorylation by imatinib and dasatinib. B-ALL mice were treated with placebo, imatinib, or dasatinib for 3 days. After the last dose, leukemic cells from the pleural effusion were analyzed by Western blotting. Each lane represents a mouse from the indicated treatment group. (D) The SRC-selective kinase inhibitor PP2 alone or with imatinib has an inhibitory effect on proliferation of BCR-ABL-transduced BM cells in Whitlock–Witte culture. The transduced cells were cultured at 1 × 105 per well in 24-well plates for 5 days, and the two drugs were added to the culture for the last 2 days. Viable cells were counted. (E) Lack of LYN, HCK, and FGR prevents CML transition to lymphoid blast crisis. Wild-type and Lyn/Hck/Fgr triple knockout BM cells from CML mice were transferred into wild-type recipient mice to assay CML transition to B-ALL by FACS analysis of GFP+ B-leukemic cells in peripheral blood. (F) Dasatinib, but not imatinib, is effective at suppressing p53-deficient leukemic cells in B-ALL mice.
Fig. 4.
Dasatinib efficiently kills highly proliferating B-leukemic cells, but not stem cells, in B-ALL mice. (A) B-ALL reappeared in most of the mice after dasatinib treatment stopped (−); the relapsed mice remained sensitive to dasatinib therapy (+). (B) A low level of GFP+ pro- or pre-B cells (<1%) persisted in dasatinib-treated mice. (C) B220+/CD43+ pro-B leukemic cells function as leukemic stem cells in B-ALL. The sorted GFP+/B220+/CD19+ cells from BM of B-ALL mice transfer B-ALL to secondary recipients after 2 months, and leukemic cells in peripheral blood are B220+/CD43+ pro-B cells.
Fig. 5.
Imatinib and dasatinib fail to eradicate BCR-ABL-expressing HSCs completely. (A) CML mice treated with imatinib and dasatinib. BMT, BM transplantation. (B) GFP+ leukemic cell counts in peripheral blood (PB) of CML mice treated with imatinib and dasatinib. (C) Comparison of the percentages of BCR-ABL-expressing HSCs (GFP+CD34−c-kit+Hoe−) in side populations (SP) of BM cells from placebo-, imatinib-, and dasatinib-treated CML mice. (D) Dasatinib inhibits BCR-ABL kinase activity in CML stem cells. BM cells from CML mice were treated with a placebo or dasatinib (100 nM) in culture for 24 h, and BCR-ABL-expressing HSCs (GFP+CD34−c-kit+Hoe−) were identified by FACS. Intracellular levels of BCR-ABL phosphorylation were determined by FACS with anti-Abl-Y412 antibody, which detects the active form of BCR-ABL. (E) A representative CML mouse treated with dasatinib for 16 weeks still contains large numbers of BCR-ABL-expressing HSCs.
Fig. 6.
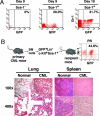
Identification of BM cell populations that function as CML stem cells. (A) BCR-ABL-transduced BM cells from B6 mice were sorted by Sca-1 MACS columns (Miltenyi Biotec, Gladbach, Germany), followed by transferring a Sca-1− or Sca-1+ population into B6 mice (1 × 105 cells per mouse; four mice per cell population group) to induce CML. GFP+ myeloid cells (Gr-1+) in peripheral blood (PB) of the mice were examined at days 9 and 19 after the induction of leukemia. All mice receiving the Sca-1+ population died of CML by day 42. (B) BCR-ABL-expressing HSCs function as CML stem cells. BM cells from CML mice in B6 background were sorted by FACS for BCR-ABL-expressing HSCs (GFP+Lin−c-kit+Sca-1+), followed by transfer into lethally irradiated B6 mice (2 × 104 cells per mouse). GFP+ myeloid cells (Gr-1+) were detected in peripheral blood. In contrast to the normal control mice, CML mice showed complete infiltration of the lungs with myeloid leukemic cells and complete disruption of follicular architecture of the spleen by infiltrating leukemic cells.
Similar articles
- [Research advance on molecular genetics of CML blast crisis].
Zhu HQ, Zhang S, Liu XL. Zhu HQ, et al. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008 Feb;16(1):217-21. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008. PMID: 18315935 Review. Chinese. - Requirement of Src kinases Lyn, Hck and Fgr for BCR-ABL1-induced B-lymphoblastic leukemia but not chronic myeloid leukemia.
Hu Y, Liu Y, Pelletier S, Buchdunger E, Warmuth M, Fabbro D, Hallek M, Van Etten RA, Li S. Hu Y, et al. Nat Genet. 2004 May;36(5):453-61. doi: 10.1038/ng1343. Epub 2004 Apr 18. Nat Genet. 2004. PMID: 15098032 - Cotreatment with vorinostat (suberoylanilide hydroxamic acid) enhances activity of dasatinib (BMS-354825) against imatinib mesylate-sensitive or imatinib mesylate-resistant chronic myelogenous leukemia cells.
Fiskus W, Pranpat M, Balasis M, Bali P, Estrella V, Kumaraswamy S, Rao R, Rocha K, Herger B, Lee F, Richon V, Bhalla K. Fiskus W, et al. Clin Cancer Res. 2006 Oct 1;12(19):5869-78. doi: 10.1158/1078-0432.CCR-06-0980. Clin Cancer Res. 2006. PMID: 17020995 - Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias.
Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, Cortes J, O'Brien S, Nicaise C, Bleickardt E, Blackwood-Chirchir MA, Iyer V, Chen TT, Huang F, Decillis AP, Sawyers CL. Talpaz M, et al. N Engl J Med. 2006 Jun 15;354(24):2531-41. doi: 10.1056/NEJMoa055229. N Engl J Med. 2006. PMID: 16775234 Clinical Trial.
Cited by
- Natural killer cell mediated missing-self recognition can protect mice from primary chronic myeloid leukemia in vivo.
Kijima M, Gardiol N, Held W. Kijima M, et al. PLoS One. 2011;6(11):e27639. doi: 10.1371/journal.pone.0027639. Epub 2011 Nov 23. PLoS One. 2011. PMID: 22132120 Free PMC article. - Deletion of the RNA-editing enzyme ADAR1 causes regression of established chronic myelogenous leukemia in mice.
Steinman RA, Yang Q, Gasparetto M, Robinson LJ, Liu X, Lenzner DE, Hou J, Smith C, Wang Q. Steinman RA, et al. Int J Cancer. 2013 Apr 15;132(8):1741-50. doi: 10.1002/ijc.27851. Epub 2012 Oct 17. Int J Cancer. 2013. PMID: 22987615 Free PMC article. - Final report of a phase II study of imatinib mesylate with hyper-CVAD for the front-line treatment of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia.
Daver N, Thomas D, Ravandi F, Cortes J, Garris R, Jabbour E, Garcia-Manero G, Borthakur G, Kadia T, Rytting M, Konopleva M, Kantarjian H, O'Brien S. Daver N, et al. Haematologica. 2015 May;100(5):653-61. doi: 10.3324/haematol.2014.118588. Epub 2015 Feb 14. Haematologica. 2015. PMID: 25682595 Free PMC article. Clinical Trial. - LSK derived LSK- cells have a high apoptotic rate related to survival regulation of hematopoietic and leukemic stem cells.
Peng C, Chen Y, Shan Y, Zhang H, Guo Z, Li D, Li S. Peng C, et al. PLoS One. 2012;7(6):e38614. doi: 10.1371/journal.pone.0038614. Epub 2012 Jun 4. PLoS One. 2012. PMID: 22675576 Free PMC article. - Critical molecular pathways in cancer stem cells of chronic myeloid leukemia.
Chen Y, Peng C, Sullivan C, Li D, Li S. Chen Y, et al. Leukemia. 2010 Sep;24(9):1545-54. doi: 10.1038/leu.2010.143. Epub 2010 Jun 24. Leukemia. 2010. PMID: 20574455 Free PMC article. Review.
References
- Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, et al. N Engl J Med. 2001;344:1031–1037. - PubMed
- Graham SM, Jorgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, Holyoake TL. Blood. 2002;99:319–325. - PubMed
- Marley SB, Deininger MW, Davidson RJ, Goldman JM, Gordon MY. Exp Hematol. 2000;28:551–557. - PubMed
- Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, Sawyers CL. Science. 2001;293:876–880. - PubMed
- Hu Y, Liu Y, Pelletier S, Buchdunger E, Warmuth M, Fabbro D, Hallek M, Van Etten RA, Li S. Nat Genet. 2004;36:453–461. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous