Structure and function of latency-associated nuclear antigen - PubMed (original) (raw)
Review
Structure and function of latency-associated nuclear antigen
S C Verma et al. Curr Top Microbiol Immunol. 2007.
Abstract
Latency-associated nuclear antigen (LANA) encoded by open reading frame 73 (ORF73) is the major latent protein expressed in all forms of KSHV-associated malignancies. LANA is a large (222-234 kDa) nuclear protein that interacts with various cellular as well as viral proteins. LANA has been classified as an oncogenic protein as it dysregulates various cellular pathways including tumor suppressor pathways associated with pRb and p53 and can transform primary rat embryo fibroblasts in cooperation with the cellular oncogene Hras. It associates with GSK-3beta, an important modulator of Wnt signaling pathway leading to the accumulation of cytoplasmic beta-catenin, which upregulates Tcf/Lef regulated genes after entering into the nucleus. LANA also blocks the expression of RTA, the reactivation transcriptional activator, which is critical for the latency to lytic switch, and thus helps in maintaining viral latency. LANA tethers the viral episomal DNA to the host chromosomes by directly binding to its cognate binding sequence within the TR region of the genome through its C terminus and to the nucleosomes through the N terminus of the molecule. Tethering to the host chromosomes helps in efficient partitioning of the viral episomes in the dividing cells. Disruptions of LANA expression led to reduction in the episomal copies of the viral DNA, supporting its role in persistence of the viral DNA. The functions known so far suggest that LANA is a key player in KSHV-mediated pathogenesis.
Figures
Fig. 1
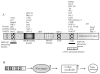
Schematic diagram of the major KSHV latency cassette. Location of the major latency transcripts cluster in KSHV genome is shown. Numbers indicate nucleotide positions, based on the database sequence NC_003409 (Russo et al. 1996). Black boldface arrows represent ORF71, 72, and 73; gray boldface arrow represents ORF74
Fig. 2
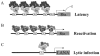
LANA shows punctuate nuclear staining. Immunofluorescence analysis showed that LANA was localized to nucleus of the different KSHV latently infected B lymphoma cell lines BCBL1, BC3, and JSC
Fig. 3
Scheme showing the structure and functional domains of LANA. LANA is a 1162-amino acid protein nuclear protein (BC-1 strain). Numbers indicate the amino acids (aa). The putative domains include an N-terminal proline-rich domain (P-rich); aspartic acid, glutamic acid repeat region (DE); glutamine-rich domain (Q-rich); leucine zipper (LZ); LANA also has two nuclear localization signals (NLS) in both the N- and C-terminal regions. The approximate position of each functional domain is shown by a black bar, corresponding to the function shown in the column on the left (Cotter and Robertson 1999; Friborg et al. 1999; Platt et al. 1999; Krithivas et al. 2000; Schwam et al. 2000; Cotter et al. 2001; Groves et al. 2001; Hyun et al. 2001; Lim et al. 2001; Krithivas et al. 2002; Mattsson et al. 2002; Shinohara et al. 2002; Borah et al. 2004; Lan et al. 2004, 2005a)
Fig. 4
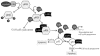
Fig. 4A–C Hypothetical model to show interplay between LANA and RTA. A During the latent infection of KSHV, LANA represses RTA expression through physical interaction with RBP-Jκ. B On reactivation of the virus, RTA can autoactivate its own expression then initiate the cascade of lytic gene expression. C In early stage of primary infection, RTA can activate LANA expression through functional association with RBP-Jκ
Fig. 5
Model showing modulation of Wnt signaling pathways through interaction with LANA. LANA binds to GSK-3β and translocates this to the nucleus, leading to the depletion of cytoplasmic GSK-3β. This results in accumulation of unphosphorylated β-catenin, which in turn translocates to the nucleus and associates with the LEF and TCF family of transcription factors, thus modulating gene expression controlled by these transcription factors
Fig.6
Modulation of pRb and p53 pathways in KSHV-infected cells. Latency-associated nuclear antigen (LANA) competes with E2F transcription factor for binding to pRb and releases the E2F transcription, which leads to the upregulation of E2F-modulated genes. LANA also interacts with p53 and blocks the p53-mediated apoptosis, thus helping in cell cycle progression and tumorigenesis
Fig. 7
Fig. 7A, B pCBP/p300. A Schematic representation of domains involved in binding to various cellular and viral proteins. LANA interacts with C/H3 domain of the protein, which is also the binding region of other viral encoded proteins including HPV E6, E1A SV40 LT, EBNA3C. B Downregulation of CBP leads to the upregulation of c-myc and cyclin D1 and thus induction of tumorigenesis
Fig. 8
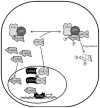
Model of KSHV episomes tethering in KSHV-infected cells. C terminus of LANA binds to its cognate sequence in TR region of KSHV genome. N terminus of LANA attaches it to nucleosome with the help of cellular proteins including histone H1, heterochromatin protein 1 (HP-1). Cellular proteins MeCp2 and DEK help in tethering of the viral genome to the host chromosome
Similar articles
- The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus manipulates the activity of glycogen synthase kinase-3beta.
Fujimuro M, Hayward SD. Fujimuro M, et al. J Virol. 2003 Jul;77(14):8019-30. doi: 10.1128/jvi.77.14.8019-8030.2003. J Virol. 2003. PMID: 12829841 Free PMC article. - Full-Length Isoforms of Kaposi's Sarcoma-Associated Herpesvirus Latency-Associated Nuclear Antigen Accumulate in the Cytoplasm of Cells Undergoing the Lytic Cycle of Replication.
Garrigues HJ, Howard K, Barcy S, Ikoma M, Moses AV, Deutsch GH, Wu D, Ueda K, Rose TM. Garrigues HJ, et al. J Virol. 2017 Nov 30;91(24):e01532-17. doi: 10.1128/JVI.01532-17. Print 2017 Dec 15. J Virol. 2017. PMID: 28978712 Free PMC article. - The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus supports latent DNA replication in dividing cells.
Hu J, Garber AC, Renne R. Hu J, et al. J Virol. 2002 Nov;76(22):11677-87. doi: 10.1128/jvi.76.22.11677-11687.2002. J Virol. 2002. PMID: 12388727 Free PMC article. - Kaposi's Sarcoma-Associated Herpesvirus Latency-Associated Nuclear Antigen: Replicating and Shielding Viral DNA during Viral Persistence.
Weidner-Glunde M, Mariggiò G, Schulz TF. Weidner-Glunde M, et al. J Virol. 2017 Jun 26;91(14):e01083-16. doi: 10.1128/JVI.01083-16. Print 2017 Jul 15. J Virol. 2017. PMID: 28446671 Free PMC article. Review. - KSHV LANA--the master regulator of KSHV latency.
Uppal T, Banerjee S, Sun Z, Verma SC, Robertson ES. Uppal T, et al. Viruses. 2014 Dec 11;6(12):4961-98. doi: 10.3390/v6124961. Viruses. 2014. PMID: 25514370 Free PMC article. Review.
Cited by
- Cyclooxygenase-2-prostaglandin E2-eicosanoid receptor inflammatory axis: a key player in Kaposi's sarcoma-associated herpes virus associated malignancies.
Paul AG, Chandran B, Sharma-Walia N. Paul AG, et al. Transl Res. 2013 Aug;162(2):77-92. doi: 10.1016/j.trsl.2013.03.004. Epub 2013 Apr 6. Transl Res. 2013. PMID: 23567332 Free PMC article. Review. - Chromatinization of the KSHV Genome During the KSHV Life Cycle.
Uppal T, Jha HC, Verma SC, Robertson ES. Uppal T, et al. Cancers (Basel). 2015 Jan 14;7(1):112-42. doi: 10.3390/cancers7010112. Cancers (Basel). 2015. PMID: 25594667 Free PMC article. Review. - Kaposi's sarcoma-associated herpesvirus-encoded LANA contributes to viral latent replication by activating phosphorylation of survivin.
Lu J, Jha HC, Verma SC, Sun Z, Banerjee S, Dzeng R, Robertson ES. Lu J, et al. J Virol. 2014 Apr;88(8):4204-17. doi: 10.1128/JVI.03855-13. Epub 2014 Jan 29. J Virol. 2014. PMID: 24478433 Free PMC article. - Interplay between Kaposi's sarcoma-associated herpesvirus and the innate immune system.
Brulois K, Jung JU. Brulois K, et al. Cytokine Growth Factor Rev. 2014 Oct;25(5):597-609. doi: 10.1016/j.cytogfr.2014.06.001. Epub 2014 Jun 21. Cytokine Growth Factor Rev. 2014. PMID: 25037686 Free PMC article. Review. - G-quadruplex-interacting compounds alter latent DNA replication and episomal persistence of KSHV.
Madireddy A, Purushothaman P, Loosbroock CP, Robertson ES, Schildkraut CL, Verma SC. Madireddy A, et al. Nucleic Acids Res. 2016 May 5;44(8):3675-94. doi: 10.1093/nar/gkw038. Epub 2016 Feb 2. Nucleic Acids Res. 2016. PMID: 26837574 Free PMC article.
References
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. - PubMed
- An FQ, Compitello N, Horwitz E, Sramkoski M, Knudsen ES, Renne R. The latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus modulates cellular gene expression and protects lymphoid cells from p16 INK4A-induced cell cycle arrest. J Biol Chem. 2005;280:3862–3874. - PubMed
- An J, Lichtenstein AK, Brent G, Rettig MB. The Kaposi sarcoma-associated herpesvirus (KSHV) induces cellular interleukin 6 expression: role of the KSHV latency-associated nuclear antigen and the AP1 response element. Blood. 2002;99:649–654. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous