Four plant Dicers mediate viral small RNA biogenesis and DNA virus induced silencing - PubMed (original) (raw)
Four plant Dicers mediate viral small RNA biogenesis and DNA virus induced silencing
Todd Blevins et al. Nucleic Acids Res. 2006.
Abstract
Like other eukaryotes, plants use DICER-LIKE (DCL) proteins as the central enzymes of RNA silencing, which regulates gene expression and mediates defense against viruses. But why do plants like Arabidopsis express four DCLs, a diversity unmatched by other kingdoms? Here we show that two nuclear DNA viruses (geminivirus CaLCuV and pararetrovirus CaMV) and a cytoplasmic RNA tobamovirus ORMV are differentially targeted by subsets of DCLs. DNA virus-derived small interfering RNAs (siRNAs) of specific size classes (21, 22 and 24 nt) are produced by all four DCLs, including DCL1, known to process microRNA precursors. Specifically, DCL1 generates 21 nt siRNAs from the CaMV leader region. In contrast, RNA virus infection is mainly affected by DCL4. While the four DCLs are partially redundant for CaLCuV-induced mRNA degradation, DCL4 in conjunction with RDR6 and HEN1 specifically facilitates extensive virus-induced silencing in new growth. Additionally, we show that CaMV infection impairs processing of endogenous RDR6-derived double-stranded RNA, while ORMV prevents HEN1-mediated methylation of small RNA duplexes, suggesting two novel viral strategies of silencing suppression. Our work highlights the complexity of virus interaction with host silencing pathways and suggests that DCL multiplicity helps mediate plant responses to diverse viral infections.
Figures
Figure 1
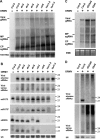
Mutations in DCL4 and RDR6 impair _Chl_-VIGS in infected tissue and abolish total _Chl_-VIGS in newly emerging leaves. (A) wt (Col-0), dcl and rdr mutant plants 1 month post-inoculation with CaLCuV::Chl. Infection causes leaf deformations and a yellow-white phenotype in Col-0 due to _Chl-_VIGS. Total DNA and RNA were isolated from pooled plants. Viral titers (B) were measured by semi-quantitative PCR on serial dilutions (5-fold each) of the DNA. 18S ribosomal DNA amplification is an internal control. (C) RNA blot hybridization was performed with column-purified total RNA (8 μg/lane). The membrane was successively hybridized with random-labeled DNA probes for ChlI, ACT2 and CaLCuV AC2/AC3 transcripts. (D) Quantitative real-time PCR was made on cDNA synthesized from RNA pools. The mean from triplicate determinations of ChlI transcript levels was normalized to each corresponding ACT2 mean; the Col-0 (mock) level was set to 1.
Figure 2
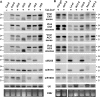
CaLCuV-derived siRNA accumulation in Arabidopsis mutants for RNA silencing pathways. Low molecular weight (LMW) RNA (10 μg/lane) from plant pools was analyzed by RNA blot hybridization. Membranes were successively hybridized with sense and antisense DNA oligo probes for the viral _ChlI_-fragment, viral AC4 region and endogenous controls (see Supplementary Table S1 for probe details). Synthetic 21 and 24 nt RNA oligos were used as markers. (A) Analysis of mock Col-0 and virus-infected Col-0, dcl- and _rdr_-mutant plants. (B) Analysis of mock Col-0, virus-infected controls (Col-gl1, La-er, Col-0), and mutants affecting miRNA accumulation (dcl1-8, dcl1-9, hen1-5, hyl1-2). U6 signal and ethidium bromide (EtBr) staining serve as loading controls.
Figure 3
CaMV-derived siRNA accumulation in Arabidopsis mutants for RNA silencing pathways. Small RNA blot analysis was performed as in Figure 2. Membranes were successively hybridized with sense and antisense DNA oligo probes for viral L1 and L2 regions of the CaMV transcript leader, TAV coding region and endogenous controls (see Supplementary Table S1 for probe details). (A) Analysis of Col-0 (mock) and virus-infected Col-0, dcl and _rdr_-mutant plants. (B) Analysis of mock Col-0, virus-infected controls (Col-gl1, La-er, Col-0), and mutants affecting miRNA accumulation (dcl1-8, dcl1-9, hen1-5, hyl1-2). EtBr staining is a loading control.
Figure 4
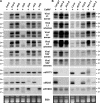
Effect of double and triple _dcl_-mutations on DNA virus infection, siRNA biogenesis and VIGS. (A) Double mutants d2d3, d2d4, d3d4 and the triple mutant d2d3d4 1 month post-inoculation with CaLCuV::Chl. (B) Quantitative real-time RT–PCR for CaLCuV::Chl-infected double and triple mutants, performed as in Figure 1D. (C) LMW RNA blot analysis of Col-0 mock and CaLCuV::Chl-infected Col-0, _dcl4, dcl_-triple and double mutants. Two RNA sample pools are shown for infected d2d3d4, d2d3 and d2d4. (D) LMW RNA blot analysis of Col-0 mock and CaMV-infected Col-0, dcl4, d2d3d4 mutants. U6 signal is a loading control.
Figure 5
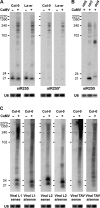
Accumulation of endogenous and viral dsRNA in CaMV-infected plants. (A) Blot hybridization analysis of total RNA (30 μg/lane) from Col-0/La-er mock and CaMV-infected plants with probes for _trans_-acting siR255 and its duplex cognate siR255*. (B) RNA (10 μg/lane) analyzed as in part A from CaMV-infected rdr6, rdr2 and dcl4 plants. (C) Membrane from part A was successively hybridized with sense and antisense probes for the CaMV transcript leader region (L1 and L2) and a coding region (TAV). Viral antisense 21, 22 and 24 nt siRNA strands of L1 and L2 regions migrate faster than sense strands due to their lower molecular weight (see Supplementary Table S1 for purine/pyrimidine contents).
Figure 6
Molecular analysis of ORMV infection in wt and RNA silencing mutant backgrounds. (A) RNA blot hybridization was performed (as in Figure 2) to detect ORMV genomic RNA (gRNA), MP and CP subgenomic RNA (sgRNA) in infected wt, dcl, rdr, hen1-5 and hyl1-2 plants. 18S RNA probe hybridization is a loading control. (B) LMW RNA blot analysis for plants in part A, using viral sense, antisense and endogenous control probes (see Supplementary Table S1 for probe details). U6 signal is a loading control. (C) Blot analysis of viral RNA in infected Col-0, d2d3d4 and d2d4 plants. EtBr staining is a loading control. (D) LMW RNA blot analysis of RNA in part C, hybridized with viral sense and antisense probes. U6 signal is a loading control.
Figure 7
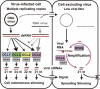
Model for VIGS and anti-viral defense based on RNA silencing. Two adjoining plant cells are shown schematically. The initially infected cell (left) contains high titers of DNA or RNA virus. Double-stranded RNA (dsRNA) arises from viral genomes independently of known silencing-related host RNA polymerases. Arrows connecting dsRNA to DCL enzymes represent the relative contribution of each DCL to viral siRNA biogenesis. For the DNA virus, every DCL digests the dsRNA into distinct size classes of siRNAs, with DCL3, DCL4 and DCL2 being favored (in that order). For RNA viruses, DCL4 is most important, with DCL2 and DCL3 compensating DCL4-deficiency (e.g. due to mutation or viral suppression). The 21 nt DCL4 product is potentially the signal required for VIGS spread. Both infectious nucleic acids and viral siRNAs move into the right-hand cell. However, the viral titer remains low, because DCL4 and RDR6 amplify incoming siRNA signal and digest viral transcripts. In this manner, VIGS would spread into meristematic tissues from which viruses are normally excluded. Viral siRNAs are stabilized by HEN1-mediated methylation.
Similar articles
- Transitivity in Arabidopsis can be primed, requires the redundant action of the antiviral Dicer-like 4 and Dicer-like 2, and is compromised by viral-encoded suppressor proteins.
Moissiard G, Parizotto EA, Himber C, Voinnet O. Moissiard G, et al. RNA. 2007 Aug;13(8):1268-78. doi: 10.1261/rna.541307. Epub 2007 Jun 25. RNA. 2007. PMID: 17592042 Free PMC article. - An antagonistic function for Arabidopsis DCL2 in development and a new function for DCL4 in generating viral siRNAs.
Bouché N, Lauressergues D, Gasciolli V, Vaucheret H. Bouché N, et al. EMBO J. 2006 Jul 26;25(14):3347-56. doi: 10.1038/sj.emboj.7601217. Epub 2006 Jun 29. EMBO J. 2006. PMID: 16810317 Free PMC article. - Plant science. Viruses face a double defense by plant small RNAs.
Waterhouse PM, Fusaro AF. Waterhouse PM, et al. Science. 2006 Jul 7;313(5783):54-5. doi: 10.1126/science.1130818. Science. 2006. PMID: 16825558 No abstract available. - Silencing suppression by geminivirus proteins.
Bisaro DM. Bisaro DM. Virology. 2006 Jan 5;344(1):158-68. doi: 10.1016/j.virol.2005.09.041. Virology. 2006. PMID: 16364747 Review. - Plant dicer-like proteins: double-stranded RNA-cleaving enzymes for small RNA biogenesis.
Fukudome A, Fukuhara T. Fukudome A, et al. J Plant Res. 2017 Jan;130(1):33-44. doi: 10.1007/s10265-016-0877-1. Epub 2016 Nov 24. J Plant Res. 2017. PMID: 27885504 Review.
Cited by
- The N-Terminal α-Helix of Potato Virus X-Encoded RNA-Dependent RNA Polymerase Is Required for Membrane Association and Multimerization.
Jiang X, Luan Y, Chai M, Yang Y, Wang Y, Deng W, Li Y, Cheng X, Wu X. Jiang X, et al. Viruses. 2022 Aug 28;14(9):1907. doi: 10.3390/v14091907. Viruses. 2022. PMID: 36146714 Free PMC article. - Combined Activity of DCL2 and DCL3 Is Crucial in the Defense against Potato Spindle Tuber Viroid.
Katsarou K, Mavrothalassiti E, Dermauw W, Van Leeuwen T, Kalantidis K. Katsarou K, et al. PLoS Pathog. 2016 Oct 12;12(10):e1005936. doi: 10.1371/journal.ppat.1005936. eCollection 2016 Oct. PLoS Pathog. 2016. PMID: 27732664 Free PMC article. - Primary and secondary siRNAs in geminivirus-induced gene silencing.
Aregger M, Borah BK, Seguin J, Rajeswaran R, Gubaeva EG, Zvereva AS, Windels D, Vazquez F, Blevins T, Farinelli L, Pooggin MM. Aregger M, et al. PLoS Pathog. 2012 Sep;8(9):e1002941. doi: 10.1371/journal.ppat.1002941. Epub 2012 Sep 27. PLoS Pathog. 2012. PMID: 23028332 Free PMC article. - A Review of Vector-Borne Rice Viruses.
Wang P, Liu J, Lyu Y, Huang Z, Zhang X, Sun B, Li P, Jing X, Li H, Zhang C. Wang P, et al. Viruses. 2022 Oct 14;14(10):2258. doi: 10.3390/v14102258. Viruses. 2022. PMID: 36298813 Free PMC article. Review. - Contribution of small RNA pathway components in plant immunity.
Seo JK, Wu J, Lii Y, Li Y, Jin H. Seo JK, et al. Mol Plant Microbe Interact. 2013 Jun;26(6):617-25. doi: 10.1094/MPMI-10-12-0255-IA. Mol Plant Microbe Interact. 2013. PMID: 23489060 Free PMC article. Review.
References
- Meins F., Jr, Si-Ammour A., Blevins T. RNA silencing systems and their relevance to plant development. Annu. Rev. Cell Dev. Biol. 2005;21:297–318. - PubMed
- Matzke M.A., Birchler J.A. RNAi-mediated pathways in the nucleus. Nature Rev. Genet. 2005;6:24–35. - PubMed
- Brodersen P., Voinnet O. The diversity of RNA silencing pathways in plants. Trends Genet. 2006;22:268–280. - PubMed
- Vaucheret H. Post-transcriptional small RNA pathways in plants: mechanisms and regulations. Genes Dev. 2006;20:759–771. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials