Cdk5 Modulation of mitogen-activated protein kinase signaling regulates neuronal survival - PubMed (original) (raw)
Cdk5 Modulation of mitogen-activated protein kinase signaling regulates neuronal survival
Ya-Li Zheng et al. Mol Biol Cell. 2007 Feb.
Abstract
Cdk5, a cyclin-dependent kinase, is critical for neuronal development, neuronal migration, cortical lamination, and survival. Its survival role is based, in part, on "cross-talk" interactions with apoptotic and survival signaling pathways. Previously, we showed that Cdk5 phosphorylation of mitogen-activated protein kinase kinase (MEK)1 inhibits transient activation induced by nerve growth factor (NGF) in PC12 cells. To further explore the nature of this inhibition, we studied the kinetics of NGF activation of extracellular signal-regulated kinase (Erk)1/2 in cortical neurons with or without roscovitine, an inhibitor of Cdk5. NGF alone induced an Erk1/2-transient activation that peaked in 15 min and declined rapidly to baseline. Roscovitine, alone or with NGF, reached peak Erk1/2 activation in 30 min that was sustained for 48 h. Moreover, the sustained Erk1/2 activation induced apoptosis in cortical neurons. Significantly, pharmacological application of the MEK1 inhibitor PD98095 to roscovitine-treated cortical neurons prevented apoptosis. These results were also confirmed by knocking down Cdk5 activity in cortical neurons with Cdk5 small interference RNA. Apoptosis was correlated with a significant shift of phosphorylated tau and neurofilaments from axons to neuronal cell bodies. These results suggest that survival of cortical neurons is also dependent on tight Cdk5 modulation of the mitogen-activated protein kinase signaling pathway.
Figures
Figure 1.
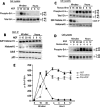
Inhibition of Cdk5 activity induced sustained Erk1/2 activation in primary cortical neurons. E18 rat embryonic cortical neurons were cultured for 7 d in B27/neurobasal medium and then treated with 50 ng/ml NGF or 20 μM roscovitine, or both and lysates prepared for analysis over a 12-h period. (A) Kinetics of Erk1/2 activation after NGF treatment. Peak Erk1/2 phosphorylation is seen at 15 min followed by a decline to baseline at 3–12 h. (B) Cdk5 immunoprecipitates of lysates (J-3 antibody) during the above-mentioned NGF activation show that the expressions of Cdk5, p35, and Cdk5 activity are constant. (C) Time course of Erk1/2 activation after treatment with roscovitine. An initial peak of activity is reached at 30 min and sustained at that level for 12 h. (D) Combined treatment, pretreated with roscovitine for 30 min and then NGF is added. Erk1/2 activation was sustained up to 12 h at a higher level than roscovitine alone. (E) Line graph shows the corresponding quantification of Erk1/2 phosphorylation in the three different treatment groups. Data represent mean ± SEM of three experiments.
Figure 2.
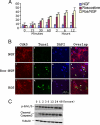
Roscovitine inhibition of Cdk5 activity induced cortical neuron apoptosis during sustained activation of Erk1/2. (A) Bar graph showing the time course of apoptosis as assayed by the TUNEL procedure. E18 rat embryonic cortical neurons were cultured for 7 d in B27/neurobasal medium and then treated with 50 ng/ml NGF, 20 μM roscovitine, or both for 12 h. Cells were fixed at different times and stained for TUNEL assay. The percentage of TUNEL-positive cells gradually increased from 1 to 12 h after roscovitine or roscovitine plus NGF treatment. Data represent mean ± SEM of three experiments. (B) Immunocytochemistry (ICC) assay of cells after 6 h of each treatment, when a high proportion of cells were apoptotic. Fixed cells were stained for Cdk5, and TUNEL fragmented, condensed apoptotic nuclei are green (b, f, and k). Images were captured using a Zeiss LSM510 laser-scanning confocal microscope and managed using Adobe Photoshop. Bar, 20 μm. (C) Treatment with roscovitine for different times induces a sustained activation of Erk1/2 at the high level for 1–48 h and causes an increasing apoptosis in cortical neurons. E18 rat embryonic cortical neurons were cultured for 7 d in B27/neurobasal medium and then treated with roscovitine. Cell lysates at different times were subjected to Western blot analysis to measure the levels of phospho-Erk1/2, cleaved caspase3, and tubulin. Sustained Erk1/2 activation correlates with elevated cleaved caspase-3 expression.
Figure 3.
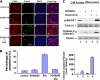
Roscovitine induced apoptosis of cortical neurons is rescued by inhibiting MEK1. E18 rat embryonic cortical neurons were cultured for 7 d in B27/neurobasal medium and then treated with 20 μM roscovitine, 50 μM PD98095, or both for 2 h and fixed for ICC assay, or lysed for Western assay. (A) Fixed cells were stained for Cdk5 and TUNEL. Most fragmented apoptotic nuclei seen after roscovitine treatment alone (j and l), whereas apoptosis was decreased when Erk1/2 activation was inhibited by PD98095 (n compared with j). Images were captured using a Zeiss LSM510 laser-scanning confocal microscope and managed using Adobe Photoshop software. Bar, 20 μm. (B) Bar graph shows the percentage of cell apoptosis. TUNEL-positive cell counts were obtained from 10 independent fields with a total of 500 cells. Results were expressed as mean ± SEM from three separate treatment groups. (C) Cleaved caspase-3 assay in neurons. Neuronal cell lysates were subjected to Western blot analysis to measure the levels of the phospho-Erk1/2, total Erk1/2, cleaved caspase-3, one of the apoptosis markers, and tubulin. High levels of phosphorylated Erk1/2 (first panel, lane 4) and apoptotic caspase-3 were expressed (third panel, lane 4), reduced in each case after treatment with the MEK1 inhibitor PD98095 (first and third panels, lane 3). Bar graph shows mean density measurement of the cleaved caspase-3. Results are expressed as mean ± SEM of three separate experiments.
Figure 4.
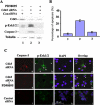
Induced apoptosis of cortical neurons by Cdk5 siRNA is rescued by inhibiting MEK1 activity. E18 rat embryonic cortical neurons were cultured for 5 d in B27/neurobasal medium and then transfected with 1) control siRNA, 2) Cdk5 siRNA, and 3) Cdk5 siRNA plus 50 μM PD98095 treatment. After 24 h of transfection, cells were fixed and/or lysed. (A) Western blots of cell lysates to measure the levels of the phospho-Erk1/2, cleaved caspase-3, and tubulin. Only in the presence of the Cdk5 siRNA were high levels of phosphorylated Erk1/2 and apoptotic caspase-3 expressed (lane 2), these were reduced in each case after treatment with the MEK1 inhibitor PD98095 (lane 3). Lanes 2 and 3 show reduced expression of cdk5 in the presence of Cdk5 siRNA. Lane 1 shows Cdk5 expression in presence of con siRNA. (B) Bar graph shows the percentage of apoptosis in each situation. Caspase-3–positive cell counts were obtained from 10 independent fields with a total of 500 cells. Results are expressed as mean ± SEM from three separate treatment groups. (C) ICC assay of cells after 24 h of transfection shows p-Erk1/2 and caspase-3–positive cells. Increased p-Erk1/2 and apoptotic cells seen only in b and d when Cdk5 activity is reduced by Cdk5 siRNA. Expressions of both were decreased when Erk1/2 activation was inhibited by PD98095 (f compared with b). Images were captured using a Zeiss LSM510 laser-scanning confocal microscope and managed using Adobe Photoshop software. Bar, 20 μm.
Figure 5.
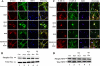
Topographic phosphorylation of cytoskeletal proteins tau and NF-H is deregulated in cortical neurons by roscovitine treatment. (A) Immunocytochemical staining of total tau and p-tau in cortical neurons treated with roscovitine with or without NGF. E18 rat embryonic cortical neurons were cultured for 7 d in B27/neurobasal medium and then treated with 1) 1× PBS as a control, 2). 50 ng/ml NGF only, 3) 20 μM roscovitine only, 4) roscovitine plus NGF, and 5) 50 μM PD98095 plus roscovitine. All groups were treated for 3 h before fixation. Control and NGF-treated neurons show normal morphology with tau (nonphospho- and phospho-tau) expression primarily in axons (a–h). Cells treated with roscovitine with or without NGF exhibit intense condensed staining in the soma with minimal staining in the axons (i–p). Cells first treated with 50 μM PD98095, followed by roscovitine show the same appearance as the control and NGF-treated cells (q–t). (B) Immunoblots of total tau and p-tau of cell lysates treated as described above. The cells were harvested and subjected Western blot by using the same phospho-tau antibody AT8 to detect phosphorylated tau and tau5 antibody for analysis total tau. (C) E18 rat hippocampal neurons, cultured for 3 d in B27/Neurobasal medium, were treated as described above, fixed, and stained for total NF-H with anti-NF-H antibody and for p-NF-H with R97 antibody. The control and NGF treatment cells display strong p-NF-H expression in axons with low expression in cell bodies (a–h). In the presence of roscovitine, however, total NF-H and p-NF-H accumulate in cell bodies, whereas axons are deleted of any expression (i–p). Cells pretreated with 50 μM PD98095 followed by roscovitine show the same appearance as the control and NGF-treated cells (q–t). (D) Immunoblots of total NF-H and p-NF-H of cell lysates treated as described above. The cells were harvested and subjected to Western blot to detect phosphorylation of NF-H with RT97 antibody and total NF-H with anti-NF-H antibody. For immunocytochemical analysis, all images were captured using a Zeiss LSM510 laser-scanning confocal microscope and managed using Adobe Photoshop software. Bar, 20 μm.
Figure 6.
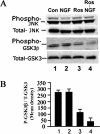
Cdk5 cross-talk with other kinase pathways, JNK and GSK3. E18 rat embryonic cortical neurons were cultured for 7 d in B27/neurobasal medium and then treated as follows: 1) nontreated, 2) 50 ng/ml NGF, 3) 20 μM roscovitine, and 4) roscovitine plus NGF. After 6-h treatment, lysates were prepared and subjected to Western blot analysis to detect the activation of JNK and GSK3 using phospho-specific antibodies. (A) JNK and GSK3 activation after NGF treatment. (B) Bar graph shows the mean densities of phosphorylated GSK3 in each situation. Results are expressed as mean ± SEM from three separate treatment groups.
Figure 7.
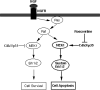
Model of Cdk5/p35 cross-talk modulation of the MAPK cascade to insure neuronal survival. In normal functioning neurons, the left side of the figure shows how Cdk5/p35 inhibits MEK1 activity to terminate the MAPK transient activation response after a short duration, thereby ensuring survival. This is based on previously reported data (Harada et al., 2001; Sharma et al., 2002). On the right side, the current data, showing the induction of sustained activation of Erk1/2 and apoptosis by roscovitine inhibition of Cdk5 activity, support the modulating role of Cdk5/p35 shown on the left side.
Similar articles
- Identification of tyrosine hydroxylase as a physiological substrate for Cdk5.
Kansy JW, Daubner SC, Nishi A, Sotogaku N, Lloyd MD, Nguyen C, Lu L, Haycock JW, Hope BT, Fitzpatrick PF, Bibb JA. Kansy JW, et al. J Neurochem. 2004 Oct;91(2):374-84. doi: 10.1111/j.1471-4159.2004.02723.x. J Neurochem. 2004. PMID: 15447670 Free PMC article. - N-acetylcysteine prevents beta-amyloid toxicity by a stimulatory effect on p35/cyclin-dependent kinase 5 activity in cultured cortical neurons.
Hsiao YH, Chen PS, Yeh SH, Lin CH, Gean PW. Hsiao YH, et al. J Neurosci Res. 2008 Sep;86(12):2685-95. doi: 10.1002/jnr.21710. J Neurosci Res. 2008. PMID: 18512759 - Differential regulation of mitogen-activated protein kinases ERK1/2 and ERK5 by neurotrophins, neuronal activity, and cAMP in neurons.
Cavanaugh JE, Ham J, Hetman M, Poser S, Yan C, Xia Z. Cavanaugh JE, et al. J Neurosci. 2001 Jan 15;21(2):434-43. doi: 10.1523/JNEUROSCI.21-02-00434.2001. J Neurosci. 2001. PMID: 11160424 Free PMC article. - Neuronal cyclin-dependent kinase 5: role in nervous system function and its specific inhibition by the Cdk5 inhibitory peptide.
Kesavapany S, Li BS, Amin N, Zheng YL, Grant P, Pant HC. Kesavapany S, et al. Biochim Biophys Acta. 2004 Mar 11;1697(1-2):143-53. doi: 10.1016/j.bbapap.2003.11.020. Biochim Biophys Acta. 2004. PMID: 15023357 Review. - Signaling pathways mediating anti-apoptotic action of neurotrophins.
Hetman M, Xia Z. Hetman M, et al. Acta Neurobiol Exp (Wars). 2000;60(4):531-45. doi: 10.55782/ane-2000-1374. Acta Neurobiol Exp (Wars). 2000. PMID: 11200182 Review.
Cited by
- Sigma-1 receptor regulates Tau phosphorylation and axon extension by shaping p35 turnover via myristic acid.
Tsai SY, Pokrass MJ, Klauer NR, Nohara H, Su TP. Tsai SY, et al. Proc Natl Acad Sci U S A. 2015 May 26;112(21):6742-7. doi: 10.1073/pnas.1422001112. Epub 2015 May 11. Proc Natl Acad Sci U S A. 2015. PMID: 25964330 Free PMC article. - Interplay between MEK-ERK signaling, cyclin D1, and cyclin-dependent kinase 5 regulates cell cycle reentry and apoptosis of neurons.
Modi PK, Komaravelli N, Singh N, Sharma P. Modi PK, et al. Mol Biol Cell. 2012 Sep;23(18):3722-30. doi: 10.1091/mbc.E12-02-0125. Epub 2012 Jul 25. Mol Biol Cell. 2012. PMID: 22833568 Free PMC article. - Novel genetic tools reveal Cdk5's major role in Golgi fragmentation in Alzheimer's disease.
Sun KH, de Pablo Y, Vincent F, Johnson EO, Chavers AK, Shah K. Sun KH, et al. Mol Biol Cell. 2008 Jul;19(7):3052-69. doi: 10.1091/mbc.e07-11-1106. Epub 2008 May 14. Mol Biol Cell. 2008. PMID: 18480410 Free PMC article. - p35 and Rac1 underlie the neuroprotection and cognitive improvement induced by CDK5 silencing.
Posada-Duque RA, López-Tobón A, Piedrahita D, González-Billault C, Cardona-Gomez GP. Posada-Duque RA, et al. J Neurochem. 2015 Jul;134(2):354-70. doi: 10.1111/jnc.13127. Epub 2015 May 4. J Neurochem. 2015. PMID: 25864429 Free PMC article.
References
- Alessandrini A., Brott B. K., Erikson R. L. Differential expression of MEK1 and MEK2 during mouse development. Cell Growth Differ. 1997;8:505–511. - PubMed
- Buee L., Bussiere T., Buee-Scherrer V., Delacourte A., Hof P. R. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Brain Res. Rev. 2000;33:95–130. - PubMed
- Cheung E. C., Slack R. S. Emerging role for ERK as a key regulator of neuronal apoptosis. Sci. STKE. 2004;2004:PE45. - PubMed
- Cheung Z. H., Ip N. Y. Cdk 5, mediator of neuronal death and survival. Neurosci. Lett. 2004;361:47–51. - PubMed
- Cheung Z. H., Fu A. K., Ip N. Y. Synaptic roles of Cdk5: implications in higher cognitive functions and neurodegenerative diseases. Neuron. 2006;50:13–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous