Androgen receptors are required for full masculinization of the ventromedial hypothalamus (VMH) in rats - PubMed (original) (raw)
Androgen receptors are required for full masculinization of the ventromedial hypothalamus (VMH) in rats
Brittany N Dugger et al. Horm Behav. 2007 Feb.
Abstract
The ventromedial hypothalamus (VMH) is one of several sexually dimorphic nuclei that regulate mating behavior, and is rich in steroid hormone receptors and aromatase activity. We looked at the contribution of the androgen receptor (AR) to the volume of the VMH in rats by measuring each of the four subdivisions of the VMH in 90 day old male, female, and XY male rats carrying a mutant AR allele (tfm), which renders animals largely unresponsive to androgens. Confirming published reports, total VMH volume was greater in wild-type males than in females (P<0.01). The mean total volume of the VMH in TFM males was intermediate, but not significantly different from either females or males (Ps>0.10). The sex difference in VMH volume was primarily accounted for by the ventrolateral subdivision (VMHvl), which in both females and TFM males was significantly smaller than in wild-type males (Ps<0.005). There was no significant sex difference in the volume of the other three subdivisions of the VMH. Neuronal somata were larger in males than females in VMHvl, central VMH (VMHc) and the dorsomedial VMH (VMHdm), with TFM males having feminine neuronal somata in the VMHdm and VMHc. These data suggest that AR plays a role during sexual differentiation of the VMH, imparting its greatest effect in the VMHvl. ARs may regulate aromatase expression or activity to affect estrogen receptor activation, or may act independently of estrogen receptors to influence VMH morphology.
Figures
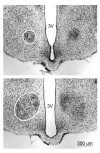
Figure 1
Thionin-stained 40µm coronal section taken at two different rostrocaudal levels of the VMH of an adult WT male. (Top) Rostral most appearance of the anterior portion (A) (Bottom) Further caudally in the same material showing the Dorsomedial, (DM) Central (C), and the Ventrolateral (VL) portions of the VMH appear. The dashed lines separate the divisions. 3V = Third ventricle.
Figure 2
Mean (± SEM) total (bilateral) volume of the VMH in adult TFM male, WT male and female rats. VMH volume is significantly greater in WT males than in females. VMH volume in TFM males is intermediate and not significantly different from WT males or females. * P < 0.02.
Figure 3
Mean (± SEM) bilateral volume of the four subdivisions of the VMH in adult TFM male rats compared with WT male and female littermates. Volume is significantly greater in WT males than in females only in the VMHvl. This subdivision is also larger in WT males than in TFM males. TFM males did not significantly differ from females in any of these measures. * P < 0.01.
Figure 4
Mean (± SEM) cross-sectional area of neuronal somata in the VMHa, VMHvl, VMHc, and the VMHdm in adult TFM male rats and WT male and female littermates. Neuronal somata are larger in males than females in the VMHvl, VMHc and the VMHdm. TFM males have significantly smaller somata than their WT male littermates in the VMHdm, and marginally smaller somata in the VMHvl and VMHc. * P < 0.05.
Similar articles
- Effects of the testicular feminization mutation (tfm) of the androgen receptor gene on BSTMPM volume and morphology in rats.
Durazzo A, Morris JA, Breedlove SM, Jordan CL. Durazzo A, et al. Neurosci Lett. 2007 May 29;419(2):168-71. doi: 10.1016/j.neulet.2007.04.033. Epub 2007 Apr 21. Neurosci Lett. 2007. PMID: 17490813 - Partial demasculinization of several brain regions in adult male (XY) rats with a dysfunctional androgen receptor gene.
Morris JA, Jordan CL, Dugger BN, Breedlove SM. Morris JA, et al. J Comp Neurol. 2005 Jun 27;487(2):217-26. doi: 10.1002/cne.20558. J Comp Neurol. 2005. PMID: 15880473 - Androgen receptors are required for full masculinization of nitric oxide synthase system in rat limbic-hypothalamic region.
Martini M, Di Sante G, Collado P, Pinos H, Guillamon A, Panzica GC. Martini M, et al. Horm Behav. 2008 Sep;54(4):557-64. doi: 10.1016/j.yhbeh.2008.05.015. Epub 2008 Jun 6. Horm Behav. 2008. PMID: 18582470 - Androgen action.
Werner R, Holterhus PM. Werner R, et al. Endocr Dev. 2014;27:28-40. doi: 10.1159/000363610. Epub 2014 Sep 9. Endocr Dev. 2014. PMID: 25247642 Review. - Selective sexual differentiation of neurone populations may contribute to sex-specific outputs of the ventromedial nucleus of the hypothalamus.
Kammel LG, Correa SM. Kammel LG, et al. J Neuroendocrinol. 2020 Jan;32(1):e12801. doi: 10.1111/jne.12801. Epub 2019 Dec 3. J Neuroendocrinol. 2020. PMID: 31605642 Free PMC article. Review.
Cited by
- Restricted effects of androgens on glucocorticoid signaling in the mouse prefrontal cortex and midbrain.
Amaya JM, Sips HCM, Viho EMG, Kroon J, Meijer OC. Amaya JM, et al. Front Endocrinol (Lausanne). 2024 Jan 18;14:1292024. doi: 10.3389/fendo.2023.1292024. eCollection 2023. Front Endocrinol (Lausanne). 2024. PMID: 38303978 Free PMC article. - Distribution of androgen receptor mRNA in the prepubertal male and female mouse brain.
Cara AL, Henson EL, Beekly BG, Elias CF. Cara AL, et al. J Neuroendocrinol. 2021 Dec;33(12):e13063. doi: 10.1111/jne.13063. Epub 2021 Dec 5. J Neuroendocrinol. 2021. PMID: 34866263 Free PMC article. - The meaning of weaning: influence of the weaning period on behavioral development in mice.
Curley JP, Jordan ER, Swaney WT, Izraelit A, Kammel S, Champagne FA. Curley JP, et al. Dev Neurosci. 2009;31(4):318-31. doi: 10.1159/000216543. Epub 2009 Jun 17. Dev Neurosci. 2009. PMID: 19546569 Free PMC article. - A role for the androgen receptor in the sexual differentiation of the olfactory system in mice.
Bodo C. Bodo C. Brain Res Rev. 2008 Mar;57(2):321-31. doi: 10.1016/j.brainresrev.2007.08.008. Epub 2007 Sep 5. Brain Res Rev. 2008. PMID: 17915335 Free PMC article. Review. - Molecular and behavioral profiling of Dbx1-derived neurons in the arcuate, lateral and ventromedial hypothalamic nuclei.
Sokolowski K, Tran T, Esumi S, Kamal Y, Oboti L, Lischinsky J, Goodrich M, Lam A, Carter M, Nakagawa Y, Corbin JG. Sokolowski K, et al. Neural Dev. 2016 May 21;11(1):12. doi: 10.1186/s13064-016-0067-9. Neural Dev. 2016. PMID: 27209204 Free PMC article.
References
- Beach FA, Buehler MG. Male rats with inherited insensitivity to androgen show reduced sexual behavior. Endocrinology. 1977;100(1):197–200. - PubMed
- Christensen LW, Nance DM, Gorski RA. Effects of hypothalamic and preoptic lesions on reproductive behavior in male rats. Brain Res Bull. 1977;2(2):137–41. - PubMed
- Cooke BM, Breedlove SM, Jordan CL. Both estrogen receptors and androgen receptors contribute to testosterone-induced changes in the morphology of the medial amygdala and sexual arousal in male rats. Horm Behav. 2003;43(2):336–46. - PubMed
- Etgen AM, Morales JC. Somatosensory stimuli evoke norepinephrine release in the anterior ventromedial hypothalamus of sexually receptive female rats. J Neuroendocrinol. 2002;14(3):213–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials