Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action - PubMed (original) (raw)
Review
Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action
M A García et al. Microbiol Mol Biol Rev. 2006 Dec.
Abstract
The double-stranded RNA-dependent protein kinase PKR is a critical mediator of the antiproliferative and antiviral effects exerted by interferons. Not only is PKR an effector molecule on the cellular response to double-stranded RNA, but it also integrates signals in response to Toll-like receptor activation, growth factors, and diverse cellular stresses. In this review, we provide a detailed picture on how signaling downstream of PKR unfolds and what are the ultimate consequences for the cell fate. PKR activation affects both transcription and translation. PKR phosphorylation of the alpha subunit of eukaryotic initiation factor 2 results in a blockade on translation initiation. However, PKR cannot avoid the translation of some cellular and viral mRNAs bearing special features in their 5' untranslated regions. In addition, PKR affects diverse transcriptional factors such as interferon regulatory factor 1, STATs, p53, activating transcription factor 3, and NF-kappaB. In particular, how PKR triggers a cascade of events involving IKK phosphorylation of IkappaB and NF-kappaB nuclear translocation has been intensively studied. At the cellular and organism levels PKR exerts antiproliferative effects, and it is a key antiviral agent. A point of convergence in both effects is that PKR activation results in apoptosis induction. The extent and strength of the antiviral action of PKR are clearly understood by the findings that unrelated viral proteins of animal viruses have evolved to inhibit PKR action by using diverse strategies. The case for the pathological consequences of the antiproliferative action of PKR is less understood, but therapeutic strategies aimed at targeting PKR are beginning to offer promising results.
Figures
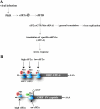
FIG. 1.
Top panel, schematic drawing of PKR activation by dsRNA-mediated dimerization, autophosphorylation, and phosphorylation of eIF-2α. R, dsRNA-binding domain., N and C, N-terminal and C-terminal lobes of kinase domain. Middle panel, crystallographic structure of PKR dimer bound to eIF-2α. Bottom panel, conformational change in eIF-2α induced by binding to PKR. Isolated eIF-2α and eIF-2α bound to PKR are in blue and red, respectively. RMSD, root mean square deviation. (The middle and bottom panels were adapted from reference with permission from Elsevier.)
FIG. 2.
(A) Impact of PKR activation and eIF-2α phosphorylation on translation. See the text for details. (B) Two examples of mRNAs whose translation is resistant to eIF-2α phosphorylation. Translation of the bona fide ATF-4 ORF is induced by low levels of functional eIF-2 (eIF-2α phosphorylation), whereas under normal conditions (high availability of eIF-2), ribosomes initiate at upstream ORFs that lead to a premature translation halt. For SV capsid mRNA, a very stable stem-loop structure downstream of initiation codon stalls the ribosome on the correct site to initiate translation. When eIF-2α is phosphorylated, SV mRNA can alternatively use the translation initiation factor 2A for delivering the Met-tRNA.
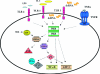
FIG. 3.
PKR is an intermediary component in TLR signaling. PKR is implicated in the LPS/TLR4-mediated pathway probably recruited by the TIRAP complex. PKR is also involved during the dsRNA/TLR3 pathway, recruited by a TAK1-containing complex. Several proteins act downstream from PKR, such as TRAF6, which has been identified downstream of PKR in the signaling cascades triggered by TLR3 and TLR4. Moreover, TRAF3 is also involved in PKR downstream events during the activation of TLR3. The activation of these pathways in turn triggers the induction of proinflammatory cytokines. RIGI/MDA5 in response to dsRNA signaling is indicated.
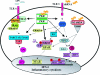
FIG. 4.
PKR acts as an activator on the signaling cascades involved during stress-activated protein kinases (MAPK) action. PKR is located upstream of MKK3 or MKK6 and MKK4 during the activation of JNK and p38 in response to several cytokines, such as IL-1 and TNF-α, and other components, such as LPS and dsRNA. Inflammatory transcription factors such as NF-κB, ATF-2, and STAT1 are finally activated.
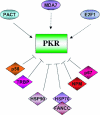
FIG. 5.
PKR is modulated by a number of cellular proteins. The PKR activator PACT, the melanoma differentiation factor MDA7, and the transcription factor E2F-1 are the only known proteins to induce PKR activation, although PACT is the best-characterized PKR activator. However, numerous cellular inhibitors of PKR have been described: p58IPK (the first cellular inhibitor reported) and the RNA-binding protein TRBP are both associated with influenza virus and HIV-1 infection. Other inhibitors of PKR action are the heat shock Hsp90/Hsp70 proteins, NPM, and the glycoprotein p67.
Similar articles
- Induction of apoptosis by the dsRNA-dependent protein kinase (PKR): mechanism of action.
Gil J, Esteban M. Gil J, et al. Apoptosis. 2000 Apr;5(2):107-14. doi: 10.1023/a:1009664109241. Apoptosis. 2000. PMID: 11232238 Review. - The dsRNA protein kinase PKR: virus and cell control.
García MA, Meurs EF, Esteban M. García MA, et al. Biochimie. 2007 Jun-Jul;89(6-7):799-811. doi: 10.1016/j.biochi.2007.03.001. Epub 2007 Mar 12. Biochimie. 2007. PMID: 17451862 Review. - NF-kappaB activation by double-stranded-RNA-activated protein kinase (PKR) is mediated through NF-kappaB-inducing kinase and IkappaB kinase.
Zamanian-Daryoush M, Mogensen TH, DiDonato JA, Williams BR. Zamanian-Daryoush M, et al. Mol Cell Biol. 2000 Feb;20(4):1278-90. doi: 10.1128/MCB.20.4.1278-1290.2000. Mol Cell Biol. 2000. PMID: 10648614 Free PMC article. - Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced protein kinase.
Gale M Jr, Katze MG. Gale M Jr, et al. Pharmacol Ther. 1998 Apr;78(1):29-46. doi: 10.1016/s0163-7258(97)00165-4. Pharmacol Ther. 1998. PMID: 9593328 Review.
Cited by
- cFLIP is critical for oligodendrocyte protection from inflammation.
Tanner DC, Campbell A, O'Banion KM, Noble M, Mayer-Pröschel M. Tanner DC, et al. Cell Death Differ. 2015 Sep;22(9):1489-501. doi: 10.1038/cdd.2014.237. Epub 2015 Jan 30. Cell Death Differ. 2015. PMID: 25633192 Free PMC article. - Influence of gut microbiota on subclinical inflammation and insulin resistance.
Carvalho BM, Saad MJ. Carvalho BM, et al. Mediators Inflamm. 2013;2013:986734. doi: 10.1155/2013/986734. Epub 2013 Jun 12. Mediators Inflamm. 2013. PMID: 23840101 Free PMC article. Review. - Targets and intracellular signaling mechanisms for deoxynivalenol-induced ribosomal RNA cleavage.
He K, Zhou HR, Pestka JJ. He K, et al. Toxicol Sci. 2012 Jun;127(2):382-90. doi: 10.1093/toxsci/kfs134. Epub 2012 Apr 5. Toxicol Sci. 2012. PMID: 22491426 Free PMC article. - Significant Variations in Double-Stranded RNA Levels in Cultured Skin Cells.
Sadeq S, Chitcharoen S, Al-Hashimi S, Rattanaburi S, Casement J, Werner A. Sadeq S, et al. Cells. 2024 Jan 25;13(3):226. doi: 10.3390/cells13030226. Cells. 2024. PMID: 38334619 Free PMC article. - Interplay between the Virus and Host in Rift Valley Fever Pathogenesis.
Terasaki K, Makino S. Terasaki K, et al. J Innate Immun. 2015;7(5):450-8. doi: 10.1159/000373924. Epub 2015 Feb 27. J Innate Immun. 2015. PMID: 25766761 Free PMC article.
References
- Abraham, N., M. L. Jaramillo, P. I. Duncan, N. Methot, P. L. Icely, D. F. Stojdl, G. N. Barber, and J. C. Bell. 1998. The murine PKR tumor suppressor gene is rearranged in a lymphocytic leukemia. Exp. Cell Res. 244:394-404. - PubMed
- Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732-738. - PubMed
- Arch, R. H., R. W. Gedrich, and C. B. Thompson. 1998. Tumor necrosis factor receptor-associated factors (TRAFs)-a family of adapter proteins that regulates life and death. Genes Dev. 12:2821-2830. - PubMed
- Balachandran, S., and G. N. Barber. 2004. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell 5:51-65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous