SRC-family kinases are activated in non-small cell lung cancer and promote the survival of epidermal growth factor receptor-dependent cell lines - PubMed (original) (raw)
SRC-family kinases are activated in non-small cell lung cancer and promote the survival of epidermal growth factor receptor-dependent cell lines
Jie Zhang et al. Am J Pathol. 2007 Jan.
Abstract
The role of Src-family kinases (SFKs) in non-small cell lung cancer (NSCLC) has not been fully defined. Here we addressed this question by examining SFK phosphorylation in NSCLC biopsy samples and using genetic and pharmacological approaches to inhibit SFK expression and activity in cultured NSCLC cells. Immunohistochemical analysis of NSCLC biopsy samples using a Tyr416 phosphorylation-specific, pan-SFK antibody revealed staining in 123 (33%) of 370 tumors. Because c-Src is known to be both an upstream activator and downstream mediator of epidermal growth factor receptor (EGFR), we next investigated SFK phosphorylation in a panel of NSCLC cell lines, including ones that depend on EGFR for survival. The EGFR-dependent NSCLC cell lines HCC827 and H3255 had increased phosphorylation of SFKs, and treatment of these cells with an SFK inhibitor (PP1 or SKI-606) induced apoptosis. PP1 decreased phosphorylation of EGFR, ErbB2, and ErbB3 and strikingly enhanced apoptosis by gefitinib, an EGFR inhibitor. HCC827 cells transfected with c-Src short hairpin RNA exhibited diminished phosphorylation of EGFR and ErbB2 and decreased sensitivity to apoptosis by PP1 or gefitinib. We conclude that SFKs are activated in NSCLC biopsy samples, promote the survival of EGFR-dependent NSCLC cells, and should be investigated as therapeutic targets in NSCLC patients.
Figures
Figure 1
SFK Tyr416 phosphorylation in NSCLC biopsy samples. A: Western blotting of H1299 cells stably transfected with dominant active mutant c-Src (DA-Src) or empty vector (H1299). B: Optimization of P-SFK immunohistochemical staining conditions so that staining was absent without primary antibody (10 Ab) or in the presence of blocking peptide (peptide). C: Examples of tumors staining positively for P-SFK (ADC, adenocarcinoma; SCC, squamous cell carcinoma; BAC, bronchioloalveolar cell carcinoma). D: Example of bronchial epithelial staining positively for P-SFK. Arrow points to a positive cell in basal layer.
Figure 2
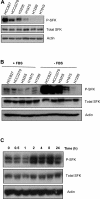
Increased SFK phosphorylation in EGFR-dependent NSCLC cell lines. A: Western blotting of P-SFK and total SFKs in NSCLC cell lines. Actin was used as a loading control. B: Serum withdrawal enhanced SFK phosphorylation in EGFR-dependent cell lines. Western analysis of NSCLC cell lines in the presence of serum (+SFK) or subjected to serum withdrawal for 48 hours (−SFK). C: SFK phosphorylation increased in a time-dependent manner after serum withdrawal. Western analysis of HCC827 cells lysed before (t = 0) or at the indicated time points after serum withdrawal. Actin was used as a loading control.
Figure 3
SFKs are required for the survival of EGFR-dependent NSCLC cells. Western blotting of NSCLC cell lines treated with PP1. A: WST-1 assays of NSCLC cells treated with PP1 or SKI-606. Values expressed relative to control cells treated with DMSO, which were set at 1. B and C: Western blotting of NSCLC cells treated with PP1 (10 μmol/L), SKI-606 (2 μmol/L), or vehicle (C). SFK inhibition was assessed by detection of P-SFK and apoptosis by caspase-3 and PARP cleavage. Actin was used as a loading control.
Figure 4
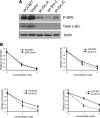
c-Src depletion in HCC827 cells diminishes sensitivity to PP1. A: Western blotting of HCC827 cells stably transfected with Src shRNA vectors (A, C, or D), empty vector (vector), or nothing (HCC827). B: WST-1 assays to examine the proliferation of transfectants (shRNA or pRS empty vector) and parental cells (HCC827) treated with PP1. Values for shRNA transfectants were expressed relative to values for parental cells (HCC827) treated with DMSO, which were set at 1. As an additional control, we compared parental cells and pRS transfectants with respect to effects of PP1, which were indistinguishable in these cells (top left).
Figure 5
SFKs regulate phosphorylation of EGFR, ErbB2, and ErbB3. A: Western analysis of ErbB family members in NSCLC cell lines treated with PP1. Actin was used as a loading control. B: Western blotting of HCC827 cells stably transfected with Src shRNA vectors (A, C, or D), empty vector (vector), or nothing (HCC827).
Figure 6
PP1 and gefitinib have synergistic effects. A: WST-1 assays to examine the proliferation of HCC827 cells and H3255 cells treated with PP1, gefitinib, or both. Values were expressed relative to control cells treated with DMSO, which were set at 1. Apoptosis detected by TUNEL assay (B) and Western blotting of PARP and cleaved caspase-3 (C) in HCC827 cells treated with gefitinib (5 nmol/L), PP1 (1 μmol/L), both, or control (DMSO). Percentages of apoptotic cells detected by TUNEL are indicated (* = %). Actin was used as a loading control in C. D: Western blotting to examine effects of gefitinib, PP1, both, or neither, on downstream mediators of EGFR and SFKs in HCC827 cells. The intensities of the bands were quantified by densitometric analysis and normalized by total ERK, total STAT3, or actin (for cyclin D1). Phosphorylation levels were expressed relative to that of control cells treated with DMSO (Con), which were set at 1.
Figure 7
c-Src depletion in HCC827 cells diminishes sensitivity to gefitinib. WST-1 assays to examine the proliferation of transfectants (shRNA or pRS empty vector) and parental cells (HCC827) treated with gefitinib. Values for shRNA transfectants were expressed relative to values for parental cells (HCC827) treated with DMSO, which were set at 1. As an additional control, we compared parental cells and pRS transfectants with respect to effects of gefitinib, which were indistinguishable in these cells (top left).
Similar articles
- SRC promotes survival and invasion of lung cancers with epidermal growth factor receptor abnormalities and is a potential candidate for molecular-targeted therapy.
Leung EL, Tam IY, Tin VP, Chua DT, Sihoe AD, Cheng LC, Ho JC, Chung LP, Wong MP. Leung EL, et al. Mol Cancer Res. 2009 Jun;7(6):923-32. doi: 10.1158/1541-7786.MCR-09-0003. Epub 2009 Jun 2. Mol Cancer Res. 2009. PMID: 19491201 - Dasatinib (BMS-354825) selectively induces apoptosis in lung cancer cells dependent on epidermal growth factor receptor signaling for survival.
Song L, Morris M, Bagui T, Lee FY, Jove R, Haura EB. Song L, et al. Cancer Res. 2006 Jun 1;66(11):5542-8. doi: 10.1158/0008-5472.CAN-05-4620. Cancer Res. 2006. PMID: 16740687 - Differential effects of gefitinib and cetuximab on non-small-cell lung cancers bearing epidermal growth factor receptor mutations.
Mukohara T, Engelman JA, Hanna NH, Yeap BY, Kobayashi S, Lindeman N, Halmos B, Pearlberg J, Tsuchihashi Z, Cantley LC, Tenen DG, Johnson BE, Jänne PA. Mukohara T, et al. J Natl Cancer Inst. 2005 Aug 17;97(16):1185-94. doi: 10.1093/jnci/dji238. J Natl Cancer Inst. 2005. PMID: 16106023 - A mechanism for SRC kinase-dependent signaling by noncatalytic receptors.
Cooper JA, Qian H. Cooper JA, et al. Biochemistry. 2008 May 27;47(21):5681-5688. doi: 10.1021/bi8003044. Epub 2008 Apr 30. Biochemistry. 2008. PMID: 18444664 Free PMC article. Review. - Src as a potential therapeutic target in non-small-cell lung cancer.
Giaccone G, Zucali PA. Giaccone G, et al. Ann Oncol. 2008 Jul;19(7):1219-1223. doi: 10.1093/annonc/mdn048. Epub 2008 Apr 3. Ann Oncol. 2008. PMID: 18388349 Review.
Cited by
- Effects of Src inhibitors on cell growth and epidermal growth factor receptor and MET signaling in gefitinib-resistant non-small cell lung cancer cells with acquired MET amplification.
Yoshida T, Okamoto I, Okamoto W, Hatashita E, Yamada Y, Kuwata K, Nishio K, Fukuoka M, Jänne PA, Nakagawa K. Yoshida T, et al. Cancer Sci. 2010 Jan;101(1):167-72. doi: 10.1111/j.1349-7006.2009.01368.x. Epub 2009 Sep 14. Cancer Sci. 2010. PMID: 19804422 Free PMC article. - Differential network analysis of ROS1 inhibitors reveals lorlatinib polypharmacology through co-targeting PYK2.
Liao Y, Remsing Rix LL, Li X, Fang B, Izumi V, Welsh EA, Monastyrskyi A, Haura EB, Koomen JM, Doebele RC, Rix U. Liao Y, et al. Cell Chem Biol. 2024 Feb 15;31(2):284-297.e10. doi: 10.1016/j.chembiol.2023.09.011. Epub 2023 Oct 16. Cell Chem Biol. 2024. PMID: 37848034 - Mechanisms of tumor resistance to EGFR-targeted therapies.
Hopper-Borge EA, Nasto RE, Ratushny V, Weiner LM, Golemis EA, Astsaturov I. Hopper-Borge EA, et al. Expert Opin Ther Targets. 2009 Mar;13(3):339-62. doi: 10.1517/14712590902735795. Expert Opin Ther Targets. 2009. PMID: 19236156 Free PMC article. Review. - A phase II trial of the Src-kinase inhibitor saracatinib after four cycles of chemotherapy for patients with extensive stage small cell lung cancer: NCCTG trial N-0621.
Molina JR, Foster NR, Reungwetwattana T, Nelson GD, Grainger AV, Steen PD, Stella PJ, Marks R, Wright J, Adjei AA. Molina JR, et al. Lung Cancer. 2014 Aug;85(2):245-50. doi: 10.1016/j.lungcan.2014.03.004. Epub 2014 May 1. Lung Cancer. 2014. PMID: 24957683 Free PMC article. Clinical Trial.
References
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. - PubMed
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. - PubMed
- Fujimoto N, Wislez M, Zhang J, Iwanaga K, Dackor J, Hanna AE, Kalyankrishna S, Cody DD, Price RE, Sato M, Shay JW, Minna JD, Peyton M, Tang X, Massarelli E, Herbst R, Threadgill DW, Wistuba II, Kurie JM. High expression of ErbB family members and their ligands in lung adenocarcinomas that are sensitive to inhibition of epidermal growth factor receptor. Cancer Res. 2005;65:11478–11485. - PubMed
- Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64:8919–8923. - PubMed
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabarbara P, Seymour L. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous