Cells infected with scrapie and Creutzfeldt-Jakob disease agents produce intracellular 25-nm virus-like particles - PubMed (original) (raw)
Cells infected with scrapie and Creutzfeldt-Jakob disease agents produce intracellular 25-nm virus-like particles
Laura Manuelidis et al. Proc Natl Acad Sci U S A. 2007.
Erratum in
- Proc Natl Acad Sci U S A. 2007 Apr 3;104(14):6090. Banquero, Nuria [corrected to Barquero, Nuria]
Abstract
We had repeatedly found approximately 25-nm-diameter virus-like particles in highly infectious brain fractions with little prion protein (PrP), and therefore we searched for similar virus-like particles in situ in infected cell lines with high titers. Neuroblastoma cells infected with the 22L strain of scrapie as well as hypothalamic GT cells infected with the FU Creutzfeldt-Jakob disease agent, but not parallel mock controls, displayed dense 25-nm virus-like particles in orthogonal arrays. These particles had no relation to abnormal PrP amyloid in situ, nor were they labeled by PrP antibodies that faithfully recognized rough endoplasmic reticulum membranes and amyloid fibrils, the predicted sites of normal and pathological intracellular PrP. Additionally, phorbol ester stimulated the production of abnormal PrP gel bands by >5-fold in infected N2a + 22L cells, yet this did not increase either the number of virus-like arrays or the infectious titer of these cells. Thus, the 25-nm infection-associated particles could not be prions. Synaptic differentiation and neurodegeneration, as well as retroviruses that populate the rough endoplasmic reticulum of neuroblastoma cells, were not required for particle production. The 25-nm particle arrays in cultured cells strongly resembled those first described in 1968 in synaptic regions of scrapie-infected brain and subsequently identified in many natural and experimental TSEs. The high infectivity of comparable, isolated virus-like particles that show no intrinsic PrP by antibody labeling, combined with their loss of infectivity when nucleic acid-protein complexes are disrupted, make it likely that these 25-nm particles are the causal TSE virions that induce late-stage PrP brain pathology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
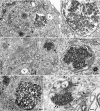
Fig. 1.
Representative virus-like arrays of 25-nm particles in N2a + 22L (A–C) and in GT+FU-CJD (D) cells. (A and B) Particles in healthy cells treated with 100 ng/ml PMA for 5 days when PrP-res was markedly increased, with higher magnifications of the same array (Right). (C) From an N2a + 22L culture without PMA, processed in parallel. (D) A comparable virus-like array in a GT+FU-CJD cell. (A Left) The membrane-bound array is distant from the nucleus (N) and sits between a mitochondrion (m) and a vacuole (V). A 100-nm-diameter IAP retroviral is seen in an RER cistern (arrow), and Golgi membranes are at g. Arrows in the corresponding magnified image point to spherical dense particles of ≈25 nm; note regions of variable compaction of particles. (B) A characteristic cluster of IAP retroviruses is seen to the right of a vacuole (V) in addition to a TSE-packed particle array adjacent to an isolated IAP (arrow). Higher magnification shows that the paracrystalline particles have sharper outlines than adjacent ribosomes. (C) Without PMA, spherical 25-nm particles also line up in an orthogonal pattern in more compact regions of N2a + 22L cells. (D) Orthogonal or paracrystalline arrangement of equivalent dense 25-nm particles in cross-section (e.g., at arrows) in infected GT cells that produce no retroviral structures is shown; a few similar 25-nm particles are seen in an adjacent swollen RER cistern (arrowhead), and these may represent a different stage of array development (see Results). (Scale bars: 100 nm.)
Fig. 2.
Relationship of PrP-res, virus-like particles, and infectivity in N2a + 22L cells grown with and without PMA. (A) Dose–response from 0 to 100 ng/ml PMA for 5 days of PrP and corresponding PrP-res amounts (+Pk lanes of whole-cell lysates treated with proteinase K as described in ref. 4). Note the strong increase in PrP-res at 100 ng/ml. CNS is brain homogenate control. MK are mock-infected cells and show no PrP-res. (B) PrP-res during continuous treatment with 100 ng/ml for 1–7 days shows the strongest response at 5 days; cells were treated in parallel and collected at sequential times. (C) Quantitative analysis of the increase in PrP-res in seven independent experiments with 75–100 ng/ml PMA (dotted line). Note the reproducible rise at 5 days (± SEM). The gray line shows the PMA-induced PrP-res increase versus a parallel nondrug control (see E) in cells that were tested for infectivity. (D) Count of virus-like 25-nm particle arrays in PMA-treated mock (uninfected N2a) control cells and in N2a + 22L cells without (22L-PMA) and with (22L+PMA) the drug. More than 250 cells were counted by EM for each sample, and the inclusion of any questionably positive arrays makes the percentage of positives overestimated. Nevertheless, there is no significant increase in the number of particle arrays with PMA. (E) Western blot of an aliquot of the mock-infected, untreated 22L, and PMA-treated 22L cells inoculated in mice for infectivity titrations. The mock cells show no PrP-res (+PK lanes), and the PMA-treated cells show an obvious increase in both PrP and PrP-res compared with their untreated counterparts. All cell lanes loaded with equal amounts of protein and infected CNS show brain-specific pattern (4). (F) Incubation time after inoculation of serial dilutions of N2a + 22L cell homogenates grown without PMA (white bars) and with PMA (gray bars). A shorter incubation time indicates a higher infectious titer. Mock-infected cells (dotted bar) at high concentrations elicited no clinical signs or neuropathology.
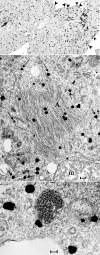
Fig. 3.
Ultrasmall immunogold labeling (silver intensified) of PrP and PrP-res in N2a + 22L cells lightly fixed and then permeabilized in situ for antigen detection. (Top) A typical low-power image where many grains are on the plasma membrane (arrowhead) and dispersed in the cytoplasm. Nuclei (N) and clusters of IAP retroviruses (arrow) are not labeled. (Middle) Grains over PrP amyloid fibers in characteristic crisscrossing bundles (A) and also over RER membranes (arrows). Label was not seen over mitochondrial cristae (m) as representatively depicted. (Bottom) PrP does not localize over the 25-nm virus-like dense particles but can be associated with the outer RER membrane. (Scale bars: 100 nm.)
Similar articles
- A 25 nm virion is the likely cause of transmissible spongiform encephalopathies.
Manuelidis L. Manuelidis L. J Cell Biochem. 2007 Mar 1;100(4):897-915. doi: 10.1002/jcb.21090. J Cell Biochem. 2007. PMID: 17044041 Review. - Viral particles are required for infection in neurodegenerative Creutzfeldt-Jakob disease.
Manuelidis L, Sklaviadis T, Akowitz A, Fritch W. Manuelidis L, et al. Proc Natl Acad Sci U S A. 1995 May 23;92(11):5124-8. doi: 10.1073/pnas.92.11.5124. Proc Natl Acad Sci U S A. 1995. PMID: 7761460 Free PMC article. - Rapid chemical decontamination of infectious CJD and scrapie particles parallels treatments known to disrupt microbes and biofilms.
Botsios S, Tittman S, Manuelidis L. Botsios S, et al. Virulence. 2015;6(8):787-801. doi: 10.1080/21505594.2015.1098804. Virulence. 2015. PMID: 26556670 Free PMC article. - Quantitative recovery of scrapie agent with minimal protein from highly infectious cultures.
Sun R, Liu Y, Zhang H, Manuelidis L. Sun R, et al. Viral Immunol. 2008 Sep;21(3):293-302. doi: 10.1089/vim.2008.0039. Viral Immunol. 2008. PMID: 18788938 Free PMC article.
Cited by
- Proliferative arrest induces neuron differentiation and innate immune responses in control and Creutzfeldt-Jakob Disease agent infected rat septal neurons.
Pagano N, Aguilar Perez G, Garcia-Milian R, Manuelidis L. Pagano N, et al. bioRxiv [Preprint]. 2024 Aug 4:2024.07.26.605349. doi: 10.1101/2024.07.26.605349. bioRxiv. 2024. PMID: 39131355 Free PMC article. Preprint. - Viruses in neurodegenerative diseases: More than just suspects in crimes.
Leblanc P, Vorberg IM. Leblanc P, et al. PLoS Pathog. 2022 Aug 4;18(8):e1010670. doi: 10.1371/journal.ppat.1010670. eCollection 2022 Aug. PLoS Pathog. 2022. PMID: 35925897 Free PMC article. Review. - Reduced Expression of Prion Protein With Increased Interferon-β Fail to Limit Creutzfeldt-Jakob Disease Agent Replication in Differentiating Neuronal Cells.
Aguilar G, Pagano N, Manuelidis L. Aguilar G, et al. Front Physiol. 2022 Feb 18;13:837662. doi: 10.3389/fphys.2022.837662. eCollection 2022. Front Physiol. 2022. PMID: 35250638 Free PMC article. - What is strain in neurodegenerative diseases?
Tian Y, Meng L, Zhang Z. Tian Y, et al. Cell Mol Life Sci. 2020 Feb;77(4):665-676. doi: 10.1007/s00018-019-03298-9. Epub 2019 Sep 17. Cell Mol Life Sci. 2020. PMID: 31531680 Free PMC article. Review. - A prokaryotic viral sequence is expressed and conserved in mammalian brain.
Yeh YH, Gunasekharan V, Manuelidis L. Yeh YH, et al. Proc Natl Acad Sci U S A. 2017 Jul 3;114(27):7118-7123. doi: 10.1073/pnas.1706110114. Epub 2017 Jun 19. Proc Natl Acad Sci U S A. 2017. PMID: 28630311 Free PMC article.
References
- Manuelidis L. Viral Immunol. 2003;16:123–139. - PubMed
- Xi YG, Ingrosso A, Ladogana A, Masullo C, Pocchiari M. Nature. 1992;356:598–601. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous