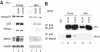Monoubiquitylation promotes mitochondrial p53 translocation - PubMed (original) (raw)
Comparative Study
Monoubiquitylation promotes mitochondrial p53 translocation
Natasha D Marchenko et al. EMBO J. 2007.
Abstract
A major function of the p53 tumor suppressor is the induction of a pleiotropic apoptotic program in response to stress through transcription-dependent and -independent mechanisms. In particular, this includes a direct apoptotic role of p53 at the mitochondria. Stress-induced p53 translocation to the mitochondria with subsequent outer membrane permeabilization is a common early component in p53-mediated apoptosis in normal and transformed cells. However, the mechanism of p53 delivery to the mitochondria remains unknown. Here, we show that the cytoplasm contains a separate and distinct p53 pool that is the major source for p53 translocation to the mitochondria upon its stress-induced stabilization. Using various manipulations that enhance or diminish p53 ubiquitylation, our data provide evidence that Mdm2-mediated monoubiquitylation of p53 greatly promotes its mitochondrial translocation and thus its direct mitochondrial apoptosis. On the other hand, p53 does not require Mdm2 as a shuttler. Upon arrival at the mitochondria, our data suggest that p53 undergoes rapid deubiquitylation by mitochondrial HAUSP via a stress-induced mitochondrial p53-HAUSP complex. This generates the apoptotically active non-ubiquitylated p53. Taken together, we propose a novel model for mitochondrial p53 targeting, whereby a distinct cytoplasmic pool of stabilized monoubiquitylated p53, generated in resting cells by basal levels of Mdm2-type ligases, is subject to a binary switch from a fate of inactivation via subsequent polyubiquitylation and degradation in unstressed cells, to a fate of activation via mitochondrial trafficking.
Figures
Figure 1
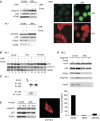
The cytoplasm contains a separate p53 pool that is the major source of translocated mitochondrial p53. (A) Mitochondria were isolated from RKO (top) and ML1 (bottom) cells by sucrose density gradients (both Arg/Pro at codon 72) treated with LMB and/or Camp for 4 h. Purified mitochondrial and crude lysates were immunoblotted and the amount of translocated p53 was detected with DO-1 antibody. Equal mitochondrial loading and purity from nuclear contamination was verified by reblotting with mthsp70 and PCNA and hsp90, respectively in (A), (D) and (E). Nuclear export blockade by LMB was verified in cells treated with LMB and immunostained for endogenous p53 and IκB. (B) Treated ML1 cells were fractionated into nuclear, cytoplasmic and crude fractions (Pierce kit) and immunoblotted as indicated. (C) Crude extract from ML1 cells were treated as indicated, immunoprecipitated with CM1 antibody (p53-specific) and immunoblotted with CRM1 antibody. (D) Immunoblot of crude lysates and mitochondria purified from RKO cells after transfection with Flag-tagged Rex for 24 h, followed by 5 μM Camp for an additional 4 h. (E, left) p53-null H1299 cells were transfected with plasmids encoding wtp53 or NLS-defective mutant p53. Immunoblot as in (A). (E, middle) Immunofluorescence with CM-1 antibody to show cytoplasmic localization of the NLS mutant. (E, right) Colony formation assays. p53-null H1299 cells were transfected with the indicated plasmids. After G418 selection for 18 days, colonies were counted.
Figure 2
Mdm2 is not required to shuttle p53 to mitochondria. (A) Equal amounts of crude lysates (crude) and purified mitochondria (mito) (4 μg) from ML1 cells treated as indicated were immunoblotted. Note that treatment of cells with proteasome inhibitors like ALLN causes extensive accumulation of mono- and polyubiquitylated p53 (not shown), explaining why ALLN alone mediates mitochondrial p53 translocation (see the text). (B) Mitochondria do not contain p53/Mdm2 complexes despite significantly induced translocated p53. Total cell lysates and purified mitochondria (400 μg each) from untreated or treated RKO cells were immunoprecipitated to detect p53–Mdm2 complexes. Similar results were seen with ML1 cells (data not shown).
Figure 3a
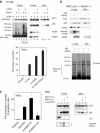
Mdm2-mediated monoubiquitylation promotes mitochondrial p53 translocation and the mitochondrial p53 apoptotic program. (A) Immunoblot of H1299 cells cotransfected with wtp53 and equal amounts of either Ubwt or UbKO. Mitochondrial loading was titrated down to the point where p53wt alone did not produce detectable translocation (lane 4) in order to show increased translocation in lanes 5, 6. (B, left) Immunoblot of RKO cells mock- or UbKO transfected. Camp-treated cells were used as control. (B, middle) RKO cells were transfected, as indicated, or left untreated. Twenty-four hours later α-amanitin was added to the culture medium where, indicated, for an additional 14 h to block transcription. Apoptosis was determined by TUNEL. (B, right) Increased apoptosis in RKO cells is accompanied by enhanced p53 monoubiquitylation after expression of Ubwt or UbKO. RKO cells were treated, as indicated, and normalized amounts of p53 were immunoblotted. (C) The fate of p53 depends on the p53:Mdm2 ratio in the cell. p53-null H1299 cells were cotransfected with 0.5 μg of p53 plasmid and decreasing concentrations of the Mdm2 plasmid. Note that whereas high concentrations of Mdm2 cause polyubiquitylation and degradation of p53, low concentrations of Mdm2 preferentially catalyze monoubiquitylation and stabilization of p53.
Figure 3b
(D) Mdm2-mediated monoubiquitylation of p53 promotes its mitochondrial targeting. Right: immunoblot of crude lysates and purified mitochondria of H1299 cells transfected with wtp53, Mdm2wt and UbKO, left: short and long DO-1 exposures of the same crude lysates +/− proteosome inhibitor ALLN to show preferential Mdm2-mediated p53 monoubiquitylation upon UbKO expression. Sample loading was normalized for the equal amount of nonubiquitilated p53. Lower panel: RKO cells were transfected, as indicated, or left untreated. Twenty-four hours later, α-amanitin was added to the medium, where indicated, for an additional 14 h to block transcription. Apoptosis was determined by TUNEL. (E) Mdm2 deficiency causes defects in mitochondrial p53 translocation and caspase 3 activation. Early passage p53−/− Mdm2−/− double knockout MEFs were retrovirally transduced with p53wt and Mdm2wt as indicated. After 36 h, crude lysates and purified mitochondria were immunoblotted as indicated, including an antibody that is specific for the activated form of caspase 3. Mitochondrial loading was normalized for equal total cellular p53 protein. Lower panel: Mdm2-mediated enhanced mitochondrial p53 translocation is associated with enhanced p53 monoubiquitylation. p53 immunoblot after long exposure. * represents nonspecific bands. (F) α-Amanitin stabilizes wtp53, induces its mitochondrial translocation, activates caspase 3 and induces apoptosis in the absence of transcription, as indicated by, for example, the p53 target gene p21. Expression of wtMdm2 enhances all of these effects, which translates into enhanced apoptosis via the mitochondrial p53 program. In contrast, expression of the catalytically inactive dominant-negative Ring-finger mutant of Mdm2 (C438L) suppresses all of these effects below the level of endogenous Mdm2 (seen with α-amanitin alone). RKO cells were transfected as indicated and 20 h later cells were treated with α-amanitin for an additional 14 h, except for parental cells. Crude and mitochondrial extracts were immunoblotted as indicated. Mitochondrial loading was normalized for equal mitochondrial protein. Apoptosis was assayed by caspase 3 cleavage and bottom by TUNEL staining. Error bars in (C), (E) and (G) are s.d. from three independent experiments.
Figure 4
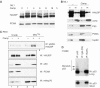
Monoubiquitylation promotes mitochondrial p53 translocation in vivo, yet the steady-state form of mitochondrial p53 is non-ubiquitylated. (A) Ubiquitin aldehyde (UbAL) is a potent deubiquitylase inhibitor in vivo. Growing cells under UbAL prevents p53 degradation and stabilizes cellular ubiquitylated p53. ML1 were cultured in the presence of 50 ng/ml UbAL for 4 h before harvesting. Crude lysates were immunoprecipitated with a mixture of the monoclonal p53 antibodies Pab1801 and DO1 and blotted for ubiquitin with polyclonal FK2 antibody. All buffers contained UbAL throughout the procedures. (B) Monoubiquitylated p53 is preferentially present at stressed mitochondria. Top: purified mitochondria and crude lysates (400 μg) from ML1 cells, treated with 5 μg Camp plus 50 ng/ml UbAL for 4 h or left untreated, were immunoprecipitated for p53 (CM1 antibody) and aliquots containing comparable amounts of non-ubiquitylated p53 in each fraction pair were immunoblotted with a pan-ubiquitin-specific antibody (P4D1). Middle: membrane was reblotted with p53-specific antibody DO1 to verify pairwise loading. Bottom: corresponding direct immunoblots from cell lysates and mitochondria used for IP. (C) Top; cytoplasmic fractions of RKO cells +/− Camp and +/− ALLN treatment were normalized for equal amounts of p53 and immunoblotted with DO1. ‘p53 short' to show equal p53 loading. Bottom; nuclear and cytoplasmic fractions of RKO cells +/− Camp and +/− ALLN. hsp90 and HDAC were used as purity controls for fractionations. (D) The steady-state form of stress-induced mitochondrial p53 is non-ubiquitylated. ML1 cells pretreated for 3 h with ALLN (50 μM), with or without UbAL (50 ng/ml), were then treated for 90 min with Camp. Crude lysates and purified mitochondria (4 μg each) were immunoblotted as indicated.
Figure 5
HAUSP is constitutively located at the mitochondria and engages in a stress-induced HAUSP–p53 complex upon p53 translocation. (A, B) HAUSP is constitutively expressed in cells and does not respond to stress. (A) Crude lysates of ML1 cells treated as indicated were immuoblotted with HAUSP antibody. PCNA is used as loading control. (B) HAUSP is also constitutively expressed at the mitochondria. Mitochondrial and crude lysates of ML1 cells treated as indicated were immunoblotted for HAUSP. A 10 μg measure of total protein per lane in (A) and (B). (C) Endogenous HAUSP forms a stress-inducible complex with translocated p53 at the mitochondria. Mitochondrial and crude lysates of RKO cells treated as indicated were immunoprecipitated for p53 (lanes 1–4) and blotted for HAUSP. Lane 5: negative control, the same amount of mitochondria were immunoprecipitated with anti-T antigen antibody. Lower panels: direct immunoblots of aliquots (5 μg each) from the IP input. (D) Mitochondrial p53, when complexed with BclX/L, is selectively non-ubiquitylated. p53–BclX/L complexes contain only non-ubiquitylated p53, whereas the total p53 pool contains detectable ubiquitylated p53 species. H1299 cells cotransfected with p53, MDM2 and BclX/L. p53 immunoprecipitations from parallel aliquots of the same lysate with 1 μg of the indicated antibodies.
Figure 6
Regulation of mitochondrial translocation of p53 in the cytoplasm. A two-step regulation of p53 enables the creation of a rapid-action binary switch. The product of the first step—monoubiquitylated p53, generated by basal levels of Mdm2-type E3 ligases—can undergo two diametrically opposed fates: (i) either degradation and inactivation by subsequent polyubiquitylation via Mdm2 or E4-type ligases if no stress occurs. (ii) Alternatively, if stress does occur, this monoubiquiylated intermediate p53 product is rapidly stabilized by stress-induced disruption of the p53–Mdm2 complex and diverted to mitochondria, hence becoming apoptoticically active. A stress-mediated decrease in Mdm2 levels might further contribute to the conversion of polyubiquitylated to monoubiquitylated p53 (Stommel and Wahl, 2004). In this way, the pre-existing and now stabilized pool of monoubiquitylated p53 mediates its first apoptotic response wave.
Similar articles
- Modulation of p53 degradation via MDM2-mediated ubiquitylation and the ubiquitin-proteasome system during reperfusion after stroke: role of oxidative stress.
Saito A, Hayashi T, Okuno S, Nishi T, Chan PH. Saito A, et al. J Cereb Blood Flow Metab. 2005 Feb;25(2):267-80. doi: 10.1038/sj.jcbfm.9600028. J Cereb Blood Flow Metab. 2005. PMID: 15678128 - The role of ubiquitination in the direct mitochondrial death program of p53.
Marchenko ND, Moll UM. Marchenko ND, et al. Cell Cycle. 2007 Jul 15;6(14):1718-23. doi: 10.4161/cc.6.14.4503. Epub 2007 May 25. Cell Cycle. 2007. PMID: 17630506 Review. - A role of HAUSP in tumor suppression in a human colon carcinoma xenograft model.
Becker K, Marchenko ND, Palacios G, Moll UM. Becker K, et al. Cell Cycle. 2008 May 1;7(9):1205-13. doi: 10.4161/cc.7.9.5756. Epub 2008 Feb 11. Cell Cycle. 2008. PMID: 18418047 Free PMC article. - Protosappanin B protects PC12 cells against oxygen-glucose deprivation-induced neuronal death by maintaining mitochondrial homeostasis via induction of ubiquitin-dependent p53 protein degradation.
Zeng KW, Liao LX, Zhao MB, Song FJ, Yu Q, Jiang Y, Tu PF. Zeng KW, et al. Eur J Pharmacol. 2015 Mar 15;751:13-23. doi: 10.1016/j.ejphar.2015.01.039. Epub 2015 Feb 2. Eur J Pharmacol. 2015. PMID: 25657114 - p53 ubiquitination: Mdm2 and beyond.
Brooks CL, Gu W. Brooks CL, et al. Mol Cell. 2006 Feb 3;21(3):307-15. doi: 10.1016/j.molcel.2006.01.020. Mol Cell. 2006. PMID: 16455486 Free PMC article. Review.
Cited by
- p53 at the endoplasmic reticulum regulates apoptosis in a Ca2+-dependent manner.
Giorgi C, Bonora M, Sorrentino G, Missiroli S, Poletti F, Suski JM, Galindo Ramirez F, Rizzuto R, Di Virgilio F, Zito E, Pandolfi PP, Wieckowski MR, Mammano F, Del Sal G, Pinton P. Giorgi C, et al. Proc Natl Acad Sci U S A. 2015 Feb 10;112(6):1779-84. doi: 10.1073/pnas.1410723112. Epub 2015 Jan 26. Proc Natl Acad Sci U S A. 2015. PMID: 25624484 Free PMC article. - A deubiquitylating complex required for neosynthesis of a yeast mitochondrial ATP synthase subunit.
Kanga S, Bernard D, Mager-Heckel AM, Erpapazoglou Z, Mattiroli F, Sixma TK, Léon S, Urban-Grimal D, Tarassov I, Haguenauer-Tsapis R. Kanga S, et al. PLoS One. 2012;7(6):e38071. doi: 10.1371/journal.pone.0038071. Epub 2012 Jun 19. PLoS One. 2012. PMID: 22723847 Free PMC article. - Minnelide/Triptolide Impairs Mitochondrial Function by Regulating SIRT3 in P53-Dependent Manner in Non-Small Cell Lung Cancer.
Kumar A, Corey C, Scott I, Shiva S, D'Cunha J. Kumar A, et al. PLoS One. 2016 Aug 8;11(8):e0160783. doi: 10.1371/journal.pone.0160783. eCollection 2016. PLoS One. 2016. PMID: 27501149 Free PMC article. - p53 improves aerobic exercise capacity and augments skeletal muscle mitochondrial DNA content.
Park JY, Wang PY, Matsumoto T, Sung HJ, Ma W, Choi JW, Anderson SA, Leary SC, Balaban RS, Kang JG, Hwang PM. Park JY, et al. Circ Res. 2009 Sep 25;105(7):705-12, 11 p following 712. doi: 10.1161/CIRCRESAHA.109.205310. Epub 2009 Aug 20. Circ Res. 2009. PMID: 19696408 Free PMC article. - The Role of p53 in Determining Mitochondrial Adaptations to Endurance Training in Skeletal Muscle.
Beyfuss K, Erlich AT, Triolo M, Hood DA. Beyfuss K, et al. Sci Rep. 2018 Oct 2;8(1):14710. doi: 10.1038/s41598-018-32887-0. Sci Rep. 2018. PMID: 30279494 Free PMC article.
References
- Appella E, Anderson CW (2001) Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem 268: 2764–2772 - PubMed
- Arima Y, Nitta M, Kuninaka S, Zhang D, Fujiwara T, Taya Y, Nakao M, Saya H (2005) Transcriptional blockade induces p53-dependent apoptosis associated with translocation of p53 to mitochondria. J Biol Chem 280: 19166–19176 - PubMed
- Bonini P, Cicconi S, Cardinale A, Vitale C, Serafino AL, Ciotti MT, Marlier LN (2004) Oxidative stress induces p53-mediated apoptosis in glia: p53 transcription-independent way to die. J Neurosci Res 75: 83–95 - PubMed
- Chipuk JE, Bouchier-Hayes L, Kuwana T, Newmeyer DD, Green DR (2005) PUMA couples the nuclear and cytoplasmic proapoptotic function of p53. Science 309: 1732–1735 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous