The deubiquitinating enzyme USP2a regulates the p53 pathway by targeting Mdm2 - PubMed (original) (raw)
Comparative Study
The deubiquitinating enzyme USP2a regulates the p53 pathway by targeting Mdm2
Lauren F Stevenson et al. EMBO J. 2007.
Abstract
Mdm2 is an E3 ubiquitin ligase that promotes its own ubiquitination and also ubiquitination of the p53 tumour suppressor. In a bacterial two-hybrid screen, using Mdm2 as bait, we identified an Mdm2-interacting peptide that bears sequence similarity to the deubiquitinating enzyme USP2a. We have established that full-length USP2a associates with Mdm2 in cells where it can deubiquitinate Mdm2 while demonstrating no deubiquitinating activity towards p53. Ectopic expression of USP2a causes accumulation of Mdm2 in a dose-dependent manner and consequently promotes Mdm2-mediated p53 degradation. This differs from the behaviour of HAUSP, which deubiquitinates p53 in addition to Mdm2 and thus protects p53 from Mdm2-mediated degradation. We further demonstrate that suppression of endogenous USP2a destabilises Mdm2 and causes accumulation of p53 protein and activation of p53. Our data identify the deubiquitinating enzyme USP2a as a novel regulator of the p53 pathway that acts through its ability to selectively target Mdm2.
Figures
Figure 1
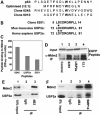
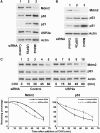
USP2a interacts with Mdm2. (A) Mdm2-interacting peptides were identified in a bacterial two-hybrid screen. p53-like sequences, from clones identified in the bacterial two-hybrid screen, aligned with the relevant portion of wild-type p53 and a p53-derived sequence optimised for binding to Mdm2. (B) Clone 5251 has homology with USP2a. Alignment of the relevant portion of clone 5251 sequence with mouse and human USP2a. (C) Peptides identified by two-hybrid screening interact with Mdm2. 16-mer biotinylated peptides derived from USP2a, clone 5251 and clone 6245 were used to capture purified Mdm2 in an ELISA. (D) EGFP-peptide fusion constructs were expressed in U-2 OS cells. Cell lysates were immunoprecipitated with α-Mdm2 antibody 4B2 and blotted for EGFP. Control (lane 1) expresses EGFP fused to an unrelated peptide. Top panel shows an EGFP blot from 7% of the IP input. *Denotes IgG light chain. (E) USP2a forms complexes with Mdm2 in vivo. USP2a was expressed in U-2 OS cells. Cell lysates were immunoprecipitated with α-Mdm2 antibody or control IgG and Mdm2 and USP2a detected by Western blotting. (F) Immunoprecipitations were performed from lysates of untransfected NTERA-2 cell with an irrelevant IgG or with anti-Mdm2 antibodies 4B2 or SMP14. Immunoprecipitates were analysed by Western blotting for Mdm2 and USP2a.
Figure 2
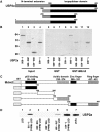
Mapping of the sites of interaction. (A) Schematic representation of wild-type USP2a and its deletions. (B) Mapping of Mdm2 binding sites in USP2a. 35S-labelled full-length USP2a (1–605) and the indicated N- and C-terminal deletions were prepared by IVT. The labelled proteins were incubated with GST or GST-Mdm2 and bound proteins were detected by autoradiography. (C) Schematic representation of wild-type Mdm2 and its deletions. (D) Mapping of USP2a binding sites in Mdm2. Full-length 35S-labelled USP2a (1–605) prepared by IVT was incubated with GST, GST-full-length Mdm2 (1–491) or the indicated GST-Mdm2 deletion mutant. Bound proteins were detected by autoradiography.
Figure 3
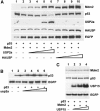
Exogenous wild-type USP2a causes accumulation of Mdm2. (A) H1299 cells were transfected with a constant amount of Mdm2 and increasing amounts (1, 3, 10 and 15 μg) of USP2a (lanes 2–5) or HAUSP (lanes 6–9). Levels of protein expression were determined by Western blotting. (B) Mdm2 accumulation is dependent on the deubiquitinating activity of USP2a. H1299 cells were transfected with Mdm2 and wild-type USP2a or a catalytic mutant of USP2a (H549A). Protein expression was analysed by Western blotting.
Figure 4
USP2a promotes Mdm2-mediated degradation of p53. (A) H1299 cells transfected with p53 (lanes 1–10) and Mdm2 where indicated (lanes 2–10) in the presence of increasing amounts (1, 3, 10 and 15 μg) of USP2a (lanes 3–6) or HAUSP (lanes 7–10) were lysed and blotted for the indicated proteins. (B) The effect of USP2a on p53 levels is dependent on the ectopic expression of Mdm2. H1299 cells were transfected with p53 and increasing amounts (1, 3, 10 and 15 μg) of USP2a. p53 and EGFP protein expression was analysed by Western blotting. (C) Mdm2 and p53 levels are not regulated by USP15. H1299 cells were transfected with Mdm2 and p53 alone (lane 1) or in the presence of increasing amounts (2, 5 and 15 μg) of USP15 (lanes 2–4). Protein expression was determined by Western blotting.
Figure 5
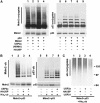
USP2a selectively deubiquitinates Mdm2 in vivo. (A) Western blots of total cell lysates. H1299 cells were transfected with p53 (lanes 1–9) and Mdm2 (lanes 1–4 and 6–9) in the presence of wild-type USP2a, a catalytic mutant of USP2a (H549A) or HAUSP. MG132 was added to the cells 6 h before harvesting in SDS–urea sample buffer. Samples were analysed by Western blotting for Mdm2 and p53. The lower panels are short exposures showing the level of unmodified protein. The upper panels are extended exposures of the same blots showing protein conjugates. To allow a direct comparison of the effects of DUB expression on the stoichiometry of Mdm2 conjugates, the amount of extracts loaded onto the SDS–PAGE gels was adjusted so that each sample contained matched amounts of unmodified Mdm2. (B) Ni2+-agarose purification of ubiquitinated proteins. H1299 cells were transfected with Mdm2 and p53 alone (lanes 1 and 5) or in the presence of His6-ubiquitin (His6-ub) (lanes 2–4 and 6–8) and the indicated DUBs. Cells were treated with MG132 for 4 h before lysis. Lysates were blotted for Mdm2 and p53 (bottom panels). Ubiquitinated proteins were purified using Ni2+-agarose and blotted for Mdm2 or p53 (upper panels). (C) USP2a expression does not alter the general pattern of ubiquitin conjugates. H1299 cells were transfected with Mdm2, p53, His6-ub and the indicated DUBS and were treated with MG132 for 4 h before lysis. Ubiquitinated proteins were purified using Ni2+-agarose and analysed by Western blotting for ubiquitin.
Figure 6
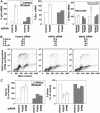
Suppression of endogenous USP2a causes accumulation of p53. (A) NTERA-2 cells transfected with the indicated siRNAs were lysed after 70 h and protein expression analysed by Western blotting. (B) LNCaP prostate cancer cells transfected with the indicated siRNAs were lysed after 48 h and protein expression was determined by Western blotting. (C) Knockdown of USP2a destabilises Mdm2 and stabilises p53. Forty-eight hours after transfection with control or USP2a synthetic siRNA duplexes, NTERA-2 cells were incubated with cycloheximide (20 μg/ml) for the indicated times. Mdm2 and p53 protein expression was determined by Western blotting. Because USP2a knockdown increases expression of p53, the Western blot shown in the left panel (control siRNA) was exposed for longer than that in the right panel (USP2a siRNA) to allow comparison of similar intensities of p53 signal. The lower panel shows quantification of the levels of Mdm2 and p53. The results are expressed as a percentage of expression in the absence of cycloheximide and are the average±s.e.m. of three experiments.
Figure 7
Knockdown of USP2a activates p53 in NTERA-2 cells. (A) USP2a suppression causes a p53-dependent increase in p53 target gene expression. Control (CMVNeo) or NTERA-2 cells expressing dominant-negative p53 (DDp53) were transfected with control or USP2a siRNA, total RNA was isolated and p53-target gene mRNA levels were quantitated by real-time PCR. mRNA levels are normalised to actin and expressed as a percentage of control (control siRNA transfection in the particular cell line). Values are means±s.d. of four experiments. (B) Suppression of USP2a causes cell death. NTERA-2 cells were transfected with the indicated siRNA. Seventy-two hours after transfection, cells were pulsed with BrdU and harvested for flow cytometry. The percentage cell-cycle distribution and the profile of a representative experiment are shown. (C) The effects of USP2a knockdown on cell-cycle progression are p53 dependent. Control (CMVNeo) or NTERA-2 cells expressing dominant-negative p53 (DDp53) were transfected with the indicated siRNAs, pulse-labelled with BrdU and harvested for FACS analysis. The results are expressed as a percentage of control (control siRNA transfection in the particular cell line) and are the average±s.d. of four experiments.
Similar articles
- MdmX is a substrate for the deubiquitinating enzyme USP2a.
Allende-Vega N, Sparks A, Lane DP, Saville MK. Allende-Vega N, et al. Oncogene. 2010 Jan 21;29(3):432-41. doi: 10.1038/onc.2009.330. Epub 2009 Oct 19. Oncogene. 2010. PMID: 19838211 - A synthetic form of frizzled 8-associated antiproliferative factor enhances p53 stability through USP2a and MDM2.
Kim J, Keay SK, You S, Loda M, Freeman MR. Kim J, et al. PLoS One. 2012;7(12):e50392. doi: 10.1371/journal.pone.0050392. Epub 2012 Dec 6. PLoS One. 2012. PMID: 23236372 Free PMC article. - ATM-mediated phosphorylations inhibit Mdmx/Mdm2 stabilization by HAUSP in favor of p53 activation.
Meulmeester E, Pereg Y, Shiloh Y, Jochemsen AG. Meulmeester E, et al. Cell Cycle. 2005 Sep;4(9):1166-70. doi: 10.4161/cc.4.9.1981. Epub 2005 Sep 29. Cell Cycle. 2005. PMID: 16082221 Review. - Up-regulated oxidized USP2a can increase Mdm2-p60-p53 to promote cell apoptosis.
Zhu H, Zhang H, Guo J, Zhang C, Zhang Q, Gao F. Zhu H, et al. Exp Cell Res. 2023 Jun 1;427(1):113597. doi: 10.1016/j.yexcr.2023.113597. Epub 2023 Apr 10. Exp Cell Res. 2023. PMID: 37044314 - Dynamics in the p53-Mdm2 ubiquitination pathway.
Brooks CL, Gu W. Brooks CL, et al. Cell Cycle. 2004 Jul;3(7):895-9. Epub 2004 Jul 2. Cell Cycle. 2004. PMID: 15254415 Review.
Cited by
- Advances in Deubiquitinating Enzyme Inhibition and Applications in Cancer Therapeutics.
Antao AM, Tyagi A, Kim KS, Ramakrishna S. Antao AM, et al. Cancers (Basel). 2020 Jun 15;12(6):1579. doi: 10.3390/cancers12061579. Cancers (Basel). 2020. PMID: 32549302 Free PMC article. Review. - ATXN3: a multifunctional protein involved in the polyglutamine disease spinocerebellar ataxia type 3.
Hernández-Carralero E, Quinet G, Freire R. Hernández-Carralero E, et al. Expert Rev Mol Med. 2024 Sep 25;26:e19. doi: 10.1017/erm.2024.10. Expert Rev Mol Med. 2024. PMID: 39320846 Free PMC article. Review. - Deubiquitinases and the new therapeutic opportunities offered to cancer.
Pfoh R, Lacdao IK, Saridakis V. Pfoh R, et al. Endocr Relat Cancer. 2015 Feb;22(1):T35-54. doi: 10.1530/ERC-14-0516. Endocr Relat Cancer. 2015. PMID: 25605410 Free PMC article. Review. - Ubiquitin-specific protease 2 regulates hepatic gluconeogenesis and diurnal glucose metabolism through 11β-hydroxysteroid dehydrogenase 1.
Molusky MM, Li S, Ma D, Yu L, Lin JD. Molusky MM, et al. Diabetes. 2012 May;61(5):1025-35. doi: 10.2337/db11-0970. Epub 2012 Mar 23. Diabetes. 2012. PMID: 22447855 Free PMC article. - SCF E3-mediated autoubiquitination negatively regulates activity of Cdc34 E2 but plays a nonessential role in the catalytic cycle in vitro and in vivo.
Scaglione KM, Bansal PK, Deffenbaugh AE, Kiss A, Moore JM, Korolev S, Cocklin R, Goebl M, Kitagawa K, Skowyra D. Scaglione KM, et al. Mol Cell Biol. 2007 Aug;27(16):5860-70. doi: 10.1128/MCB.01555-06. Epub 2007 Jun 11. Mol Cell Biol. 2007. PMID: 17562869 Free PMC article.
References
- Bottger A, Bottger V, Sparks A, Liu WL, Howard SF, Lane DP (1997) Design of a synthetic Mdm2-binding mini protein that activates the p53 response in vivo. Curr Biol 7: 860–869 - PubMed
- Bottger V, Bottger A, Howard SF, Picksley SM, Chene P, Garcia-Echeverria C, Hochkeppel HK, Lane DP (1996) Identification of novel mdm2 binding peptides by phage display. Oncogene 13: 2141–2147 - PubMed
- Brummelkamp TR, Nijman SM, Dirac AM, Bernards R (2003) Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature 424: 797–801 - PubMed
- Cummins JM, Rago C, Kohli M, Kinzler KW, Lengauer C, Vogelstein B (2004) Tumour suppression: disruption of HAUSP gene stabilizes p53. Nature 428, 1 p following 486 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous