Activation of Notch signaling in tumorigenesis of experimental pancreatic cancer induced by dimethylbenzanthracene in mice - PubMed (original) (raw)
Activation of Notch signaling in tumorigenesis of experimental pancreatic cancer induced by dimethylbenzanthracene in mice
Kenji Kimura et al. Cancer Sci. 2007 Feb.
Abstract
To establish pancreatic cancer in mice, dimethylbenzanthracene (DMBA) was administered into mice pancreata. The formation of tubular complex lesions was found in the pancreatic sections from 2 weeks after DMBA treatment. Abnormal tubular complex formations with ductal metaplasia were found from 1 month after the administration. By 3 months after DMBA injection into the pancreas, 6 of 10 mice showed visually recognizable tumors with precursor lesions of various types of cell atypia. In contrast, there were no visually or histologically detectable tumors in the placebo-treated animals. The expression profiles of smad 4, cyclin D1 and p53 in the DMBA-induced tumors were similar to those of human pancreatic cancer, suggesting that this would be a useful mouse model for studying the morphological and molecular mechanisms involved in pancreatic carcinogenesis. Immunohistochemical study using specific antibodies revealed that Notch-1 and Hes-1 were expressed in lesions ranging from tubular complexes to carcinoma in these chemically induced pancreatic tumors. Semiquantitative reverse transcription-polymerase chain reaction with microdissection demonstrated that Notch-1 expression was continuous from precursor lesions to carcinoma cells, whereas Pdx-1 expression was attenuated in carcinoma cells compared to precursor lesions. In addition, inhibition of the Notch signaling pathway by the gamma-secretase inhibitor N-(N-[3,5-difluorophenacetyl]-L-alanyl)-S-phenylglycine t-butyl ester reduced pancreatic cancer cell growth. Therefore, Notch signaling is required to form the tubular complexes and its continuous activation might lead to the transition from tubular complexes to premalignant or malignant lesions and carcinoma cell development in the pancreas.
Figures
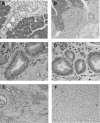
Figure 1
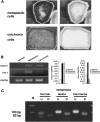
Histological findings of pancreas 2 weeks, 2 months and 3 months following the administration of dimethylbenzanthracene (DMBA). (A) Two weeks after injection, tubular complexes were present focally among acinar cells (H&E, original magnification × 200). (B) Ductal metaplasia adjacent to normal acinar tissues at 2 months after DMBA injection (H&E, original magnification × 50). (C) and (D) are high‐power views of mild dysplastic lesions in part (B). Mucin‐producing epithelial cells were seen within the metaplasic lesions (H&E, original magnification × 200). (E) Adenocarcinoma lesions accompanied by dysplastic lesions were seen in the pancreas 2 months after the administration of DMBA. (F) Ductal adenocarcinoma (sarcomatoid carcinoma) developed 3 months after the implantation of DMBA (H&E, original magnification × 200).
Figure 2
The administration of dimethylbenzanthracene (DMBA) produced a tumor in the pancreatic tail after 3 months. (A and B) A pancreatic tumor (∼10 mm in diameter) invading neighboring organs was observed clearly; B is the extracted tissues. (C) Pancreatic tumor (circled) invaded the spleen and colon (H&E, original magnification × 100).
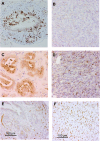
Figure 3
Pancreatic tumors developed with the carcinogen dimethylbenzanthracene originated from epithelial cells. Positive immunoreactivity of cytokeratin (left in upper panel), and Periodic acid schiff (PAS) stain‐positive cells were detected in carcinoma cells (left in middle panel) whereas immunostaining of vimentin was not seen in these lesions (right in upper panel). Intense positive staining of nestin was also observed in carcinoma cells (right middle panel). Chymotrypsin immunoreactivity was not seen in carcinoma cells (left lower panel) (original magnification × 200).
Figure 4
Smad4, cyclin D1 and p53 expression in the chemically induced pancreatic tumors. (A) Intense nuclear expression of smad4 was found in metaplastic lesions. (B) In contrast, the expression of smad4 had disappeared in carcinoma cells. Nuclear expression of cyclin D1 was found more frequently in carcinoma cells (D) but very rarely in metaplastic lesions (C). Nuclear accumulation of p53 is abundant in carcinoma cells (F) but not in metaplastic lesions (E). Although a VECTOR MOM immunodetection kit was used to reduce the background of mouse p53 antibody on mouse tissue, the stromal cells around metaplastic ductal lesions showed background staining to some extent (E). (Original magnification × 200.)
Figure 5
Activation of the Notch pathway in pancreatic carcinogenesis of mice. (C) Notch‐1 and (D) Hes‐1 were expressed in the cytoplasm and nuclei of metaplastic lesions whereas no positive staining of these proteins was seen in normal pancreas (A, Notch‐1; B, Hes‐1). Dominant nuclear expression of (E) Notch‐1 and (F) Hes‐1 was observed in sarcomatoid carcinoma cells. (Original magnification × 200.)
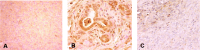
Figure 6
Pdx‐1 expression in tumors developed in dimethylbenzanthracene (DMBA)‐injected pancreas. Strong expression of Pdx‐1 protein was found in (B) metaplastic lesions and (C) heterogeneously in carcinoma lesions, but (A) this protein expression was not observed in normal pancreas. The area indicated by the arrowhead shows the expression of Pdx‐1, which was absent in other areas. In addition, the dark brown staining was stronger in the metaplastic duct compared to carcinoma cells. (Original magnification × 200.)
Figure 7
RNA expression of Notch‐1 and Pdx‐1, and K‐ras gene mutation in normal, metaplastic and carcinoma lesions in the dimethylbenzanthracene (DMBA)‐administered pancreas. (A) RNA was extracted from the microdissected lesions. Toluidine blue‐stained metaplastic (upper left) and carcinoma lesions (lower left) were cut by the laser and blown off by the large‐capacity laser, respectively (upper right and lower left right) and recovered in lysis buffer. (B) Extracted RNA from microdissected lesions was subjected to reverse transcription–polymerase chain reaction (PCR). Notch‐1 expression was detected consistently in precursor metaplastic lesions and carcinoma lesions but not in normal duct cells. In contrast, Pdx‐1 expression was more intense in precursor lesions than in carcinoma cells. Normal duct cells did not show mRNA expression of Pdx‐1. The PCR products were compared and normalized to glyceraldehyde‐3‐phosphate dehydrogenase. (C) Restriction fragment length polymorphism analysis of microdissected samples for codon 12 K‐ras mutation. Any mutation in codon 12 eliminates the restriction site so the mutated allele is resistant to digestion with _BstN_I. The PCR products from microdissected normal, metaplastic and carcinoma lesions were treated with _BstN_I. No resistance to digestion was seen in these lesions. D, digested samples; M, molecular marker; U, undigested samples.
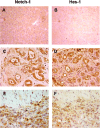
Figure 8
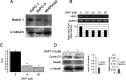
Inhibition of Notch signaling reduced pancreatic cancer growth. (A) Western blot analysis showed Notch‐1 protein expression in human pancreatic cancer cell lines (AsPC‐1, BxPC3 and MIAPaca2). α‐Tubulin was used as an internal control. (B) Treatment with _N_‐(_N_‐[3,5‐difluorophenacetyl]‐
l
‐alanyl)‐_S_‐phenylglycine _t_‐butyl ester (DAPT) reduced Hes‐1 mRNA expression in BxPC3 cells. The intensity of the band corresponding to Hes‐1 after treatment with DAPT was decreased in a dose‐dependent manner (upper panel). Real‐time reverse transcription–polymerase chain reaction also indicated the downregulation of Hes‐1 by DAPT (lower panel). (C) BxPC3 cells were treated with 10–20 ∝M DAPT for 72 h. MTT assay showed a 75–82% reduction in cell growth after DAPT treatment compared to control (**P < 0.01). (D) Western blot analysis showed downregulation of cyclin D1 and nestin expression in BxPC3 cells after incubation with DAPT for 48 h. The bands obtained were subjected to densitometry analysis and compared and normalized to α‐tubulin using Scion Image Software.
Similar articles
- Transition to pancreatic cancer in response to carcinogen.
Bockman DE. Bockman DE. Langenbecks Arch Surg. 2008 Jul;393(4):557-60. doi: 10.1007/s00423-007-0274-2. Epub 2008 Jan 12. Langenbecks Arch Surg. 2008. PMID: 18189145 Review. - NFATc1 Links EGFR Signaling to Induction of Sox9 Transcription and Acinar-Ductal Transdifferentiation in the Pancreas.
Chen NM, Singh G, Koenig A, Liou GY, Storz P, Zhang JS, Regul L, Nagarajan S, Kühnemuth B, Johnsen SA, Hebrok M, Siveke J, Billadeau DD, Ellenrieder V, Hessmann E. Chen NM, et al. Gastroenterology. 2015 May;148(5):1024-1034.e9. doi: 10.1053/j.gastro.2015.01.033. Epub 2015 Jan 23. Gastroenterology. 2015. PMID: 25623042 Free PMC article. - Krüppel-like Factor 5, Increased in Pancreatic Ductal Adenocarcinoma, Promotes Proliferation, Acinar-to-Ductal Metaplasia, Pancreatic Intraepithelial Neoplasia, and Tumor Growth in Mice.
He P, Yang JW, Yang VW, Bialkowska AB. He P, et al. Gastroenterology. 2018 Apr;154(5):1494-1508.e13. doi: 10.1053/j.gastro.2017.12.005. Epub 2017 Dec 15. Gastroenterology. 2018. PMID: 29248441 Free PMC article. - Nicotine promotes initiation and progression of KRAS-induced pancreatic cancer via Gata6-dependent dedifferentiation of acinar cells in mice.
Hermann PC, Sancho P, Cañamero M, Martinelli P, Madriles F, Michl P, Gress T, de Pascual R, Gandia L, Guerra C, Barbacid M, Wagner M, Vieira CR, Aicher A, Real FX, Sainz B Jr, Heeschen C. Hermann PC, et al. Gastroenterology. 2014 Nov;147(5):1119-33.e4. doi: 10.1053/j.gastro.2014.08.002. Epub 2014 Aug 12. Gastroenterology. 2014. PMID: 25127677 - Notch signaling pathway in pancreatic tumorigenesis.
Chung WC, Xu K. Chung WC, et al. Adv Cancer Res. 2023;159:1-36. doi: 10.1016/bs.acr.2023.02.001. Epub 2023 Feb 28. Adv Cancer Res. 2023. PMID: 37268393 Review.
Cited by
- Reg3g Promotes Pancreatic Carcinogenesis in a Murine Model of Chronic Pancreatitis.
Yin G, Du J, Cao H, Liu X, Xu Q, Xiang M. Yin G, et al. Dig Dis Sci. 2015 Dec;60(12):3656-68. doi: 10.1007/s10620-015-3787-5. Epub 2015 Jul 17. Dig Dis Sci. 2015. PMID: 26182900 - Targeting epigenetic regulation of miR-34a for treatment of pancreatic cancer by inhibition of pancreatic cancer stem cells.
Nalls D, Tang SN, Rodova M, Srivastava RK, Shankar S. Nalls D, et al. PLoS One. 2011;6(8):e24099. doi: 10.1371/journal.pone.0024099. Epub 2011 Aug 31. PLoS One. 2011. PMID: 21909380 Free PMC article. - Morphogenesis of pancreatic cancer: role of pancreatic intraepithelial neoplasia (PanINs).
Koorstra JB, Feldmann G, Habbe N, Maitra A. Koorstra JB, et al. Langenbecks Arch Surg. 2008 Jul;393(4):561-70. doi: 10.1007/s00423-008-0282-x. Epub 2008 Feb 19. Langenbecks Arch Surg. 2008. PMID: 18283486 Free PMC article. Review. - The pathobiological impact of cigarette smoke on pancreatic cancer development (review).
Wittel UA, Momi N, Seifert G, Wiech T, Hopt UT, Batra SK. Wittel UA, et al. Int J Oncol. 2012 Jul;41(1):5-14. doi: 10.3892/ijo.2012.1414. Epub 2012 Mar 23. Int J Oncol. 2012. PMID: 22446714 Free PMC article. Review. - NOTCH inhibition and glucocorticoid therapy in T-cell acute lymphoblastic leukemia.
Real PJ, Ferrando AA. Real PJ, et al. Leukemia. 2009 Aug;23(8):1374-7. doi: 10.1038/leu.2009.75. Epub 2009 Apr 9. Leukemia. 2009. PMID: 19357700 Free PMC article. Review.
References
- Hruban RH, Adsay NV, Albores‐Saavedra J et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol 2001; 25: 579 – 86. - PubMed
- Miyamoto Y, Maitra A, Ghosh B et al. Notch mediates TGFα‐induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell 2003; 3: 565 – 76. - PubMed
- Bockman DE, Guo J, Buchler P, Muller MW, Bergmann F, Friess H. Origin and development of the precursor lesions in experimental pancreatic cancer in rats. Lab Invest 2003; 83: 853 – 9. - PubMed
- Artavanis‐Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science 1999; 284: 770 – 6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous