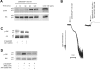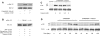Regulation and functional consequences of ADP receptor-mediated ERK2 activation in platelets - PubMed (original) (raw)
Regulation and functional consequences of ADP receptor-mediated ERK2 activation in platelets
Analia Garcia et al. Biochem J. 2007.
Abstract
We have previously shown that ADP-induced thromboxane generation in platelets requires signalling events from the G(q)-coupled P2Y1 receptor (platelet ADP receptor coupled to stimulation of phospholipase C) and the G(i)-coupled P2Y12 receptor (platelet ADP receptor coupled to inhibition of adenylate cyclase) in addition to outside-in signalling. While it is also known that extracellular calcium negatively regulates ADP-induced thromboxane A2 generation, the underlying mechanism remains unclear. In the present study we sought to elucidate the signalling mechanisms and regulation by extracellular calcium of ADP-induced thromboxane A2 generation in platelets. ERK (extracllular-signal-regulated kinase) 2 activation occurred when outside-in signalling was blocked, indicating that it is a downstream event from the P2Y receptors. However, blockade of either P2Y1 or the P2Y12 receptors with corresponding antagonists completely abolished ERK phosphorylation, indicating that both P2Y receptors are required for ADP-induced ERK activation. Inhibitors of Src family kinases or the ERK upstream kinase MEK [MAPK (mitogen-activated protein kinase)/ERK kinase] abrogated ADP-induced ERK phosphorylation and thromboxane A2 generation. Finally ADP- or G(i)+G(z)-induced ERK phosphorylation was blocked in the presence of extracellular calcium. The present studies show that ERK2 is activated downstream of P2Y receptors through a complex mechanism involving Src kinases and this plays an important role in ADP-induced thromboxane A2 generation. We also conclude that extracellular calcium blocks ADP-induced thromboxane A2 generation through the inhibition of ERK activation.
Figures
Figure 1. Role of P2Y receptors in 2MeSADP-induced phosphorylation of ERK in platelets
Washed and aspirin-treated human platelets were stimulated with 100 nM 2MeSADP for different time points or 100 ng/ml convulxin (CVX) with stirring as indicated at 37 °C and ERK phosphorylation was measured using Western blot analysis using a phospho-ERK-specific antibody (A). Washed human platelets, without aspirin-treatment, were stimulated with 2MeSADP for 60 s in stirring conditions in the presence or absence of the GPIIb/IIIa antagonist, GR 144053 without added calcium. Platelet aggregation (B) and ERK phosphorylation (C) were measured by lumi-aggregometry and Western blot analysis respectively. Washed and aspirin-treated platelets were stimulated with 100 nM 2MeSADP in the presence of a P2Y1 receptor antagonist, MRS2179, or a P2Y12 receptor antagonist, AR-C69931MX, and ERK phosphorylation was measured by Western blot analysis (D). Total ERK antibodies were used in the Western blot analysis to ensure similar protein loading in all lanes. The data are representative of experiments performed using platelets from at least three different donors.
Figure 2. Effect of MEK inhibition on ADP-induced platelet functional responses
Washed human platelets were stimulated with 2MeSADP (100 nM) or ADP (10 μM), without added calcium, in the presence of the MEK kinase inhibitor, U0126 (10 μM) or its inactive analogue, U0124 (10 μM) as indicated. Pre-incubation times for U0126 and U0124 were 10 min at 37 °C. Platelet aggregation (A), platelet secretion (B) and thromboxane A2 generation (C) were measured as described in the Experimental section. Washed platelets treated with aspirin were stimulated with 2MeSADP and platelet aggregation was measured (D). All the above data are representative of experiments performed using platelets from at least three different donors. The maximum level of thromboxane seen upon stimulation with ADP was 4452 pg/ml. TXB2, thromboxane B2.
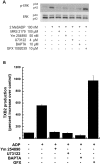
Figure 3. Role of Gq pathway signalling mediators on ADP-induced ERK phosphorylation and thromboxane A2 generation
Washed human platelets, treated with either aspirin or vehicle, were stimulated, without added calcium, with 2MeSADP (100 nM) or ADP (10 μM) in the presence or absence of a Gq blocker (YM-254890), a PLC inhibitor (U73122), a calcium chelator (BAPTA) or a PKC inhibitor (GFX) as indicated in the Figure. All of the inhibitors were incubated for 10 min at 37 °C prior to stimulation. ERK phosphorylation in aspirin-treated platelets (A) and thromboxane A2 generation in vehicle-treated platelets (B) were measured by Western blot analysis and ELISA respectively. All of the above data are representative of experiments performed using platelets from at least three different donors. TXB2, thromboxane B2.
Figure 4. Role of ERK in Gi+Gz-mediated activation of platelets
Washed and aspirin-treated human platelets were stimulated with 10 μM ADP alone or with 10 μM ADP+100 μM MRS2179+10 μM adrenaline (epinephrine) for 1 min, without added calcium, with stirring at 37 °C, and ERK phosphorylation was measured using Western blot analysis using a phospho-ERK-specific antibody (A). Total ERK antibodies were used in the Western blot analysis to ensure similar protein loading in all lanes. The data are representative of experiments performed using platelets from at least three different donors. (B) Washed human platelets were stimulated with 10 μM ADP+100 μM MRS-2179+10 μM adrenaline in the presence of the MEK kinase inhibitor, U0126 (10 μM) or its inactive analogue, U0124 (10 μM) as indicated. Pre-incubation times for U0126 and U0124 were 10 min at 37 °C. Thromboxane A2 generation was measured as described in the Experimental section and normalized to 100%. All of the above data are generated using platelets from at least three different donors. TXB2, thromboxane B2.
Figure 5. Effect of Src kinase inhibition on ADP-mediated platelet aggregation, ERK phosphorylation and thromboxane A2 generation
Washed human platelets, without aspirin treatment, were stimulated with 2MeSADP (100 nM) or ADP (10 μM) for 60 s, without added calcium, in the presence or absence of the Src kinase inhibitor (PP2). The inactive analogue, PP3, was used as a negative control. Both PP3 and PP2 were pre-incubated at 37 °C for 10 min prior to stimulation. Platelet aggregation (A), ERK phosphorylation (B), and thromboxane A2 (C) were measured by lumiaggregometry, Western blot analysis and ELISA respectively. All of the above data are representative of experiments performed using platelets from at least three different donors. TXB2, thromboxane B2.
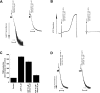
Figure 6. Effect of extracellular calcium on (A) 2MeSADP- or (B) Gi+Gz-induced ERK phosphorylation
Washed and aspirin-treated human platelets were resuspended in Tyrodes buffer with or without 2 mM extracellular calcium, and stimulated with 100 nM 2MeSADP (A) or 10 μM ADP+100 μM MRS-2179+10 μM adrenaline (epinephrine) (B) with stirring at 37 °C, and ERK phosphorylation was measured by Western blot analysis. The effect of various concentrations of extracellular calcium (C) and the time course of ERK2 phosphorylation in the presence or absence of extracellular calcium (D) were measured. Each Western blot is representative of experiments performed using platelets from at least three different donors.
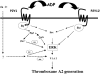
Figure 7. Model depicting the mechanism of activation and functional role of ERK in ADP-induced thromboxane A2 generation
Signalling through both the P2Y1 and the P2Y12 receptors is essential for ADP-induced ERK2 activation in platelets. Activation of PLC and subsequent intracellular calcium increases are necessary for ADP-induced ERK2 activation. Src activation occurring downstream of the P2Y receptor activation is required for ADP-induced ERK activation. Extracellular calcium and specific PKC isoform(s) negatively regulate ADP-induced ERK2 phosphorylation and thromboxane generation through as yet undefined mechanisms. The identity of the specific PKC isoform(s) mediating this negative regulation is yet to be identified.
Similar articles
- ADP secretion and subsequent P2Y12 receptor signalling play a crucial role in thrombin-induced ERK2 activation in human platelets.
Fälker K, Lange D, Presek P. Fälker K, et al. Thromb Haemost. 2004 Jul;92(1):114-23. doi: 10.1160/TH03-12-0729. Thromb Haemost. 2004. PMID: 15213852 - P2Y12 receptor-mediated potentiation of thrombin-induced thromboxane A2 generation in platelets occurs through regulation of Erk1/2 activation.
Shankar H, Garcia A, Prabhakar J, Kim S, Kunapuli SP. Shankar H, et al. J Thromb Haemost. 2006 Mar;4(3):638-47. doi: 10.1111/j.1538-7836.2006.01789.x. J Thromb Haemost. 2006. PMID: 16460446 - Role of phosphoinositide 3-kinase beta in platelet aggregation and thromboxane A2 generation mediated by Gi signalling pathways.
Garcia A, Kim S, Bhavaraju K, Schoenwaelder SM, Kunapuli SP. Garcia A, et al. Biochem J. 2010 Jul 15;429(2):369-77. doi: 10.1042/BJ20100166. Biochem J. 2010. PMID: 20441566 - The role of ADP receptors in platelet function.
Murugappa S, Kunapuli SP. Murugappa S, et al. Front Biosci. 2006 May 1;11:1977-86. doi: 10.2741/1939. Front Biosci. 2006. PMID: 16368572 Review. - Platelet receptors for adenine nucleotides and thromboxane A2.
Murugappan S, Shankar H, Kunapuli SP. Murugappan S, et al. Semin Thromb Hemost. 2004 Aug;30(4):411-8. doi: 10.1055/s-2004-833476. Semin Thromb Hemost. 2004. PMID: 15354262 Review.
Cited by
- IL-17A facilitates platelet function through the ERK2 signaling pathway in patients with acute coronary syndrome.
Zhang S, Yuan J, Yu M, Fan H, Guo ZQ, Yang R, Guo HP, Liao YH, Wang M. Zhang S, et al. PLoS One. 2012;7(7):e40641. doi: 10.1371/journal.pone.0040641. Epub 2012 Jul 11. PLoS One. 2012. PMID: 22808218 Free PMC article. - The protein tyrosine phosphatase PTPN7 is a negative regulator of ERK activation and thromboxane generation in platelets.
Inamdar VV, Reddy H, Dangelmaier C, Kostyak JC, Kunapuli SP. Inamdar VV, et al. J Biol Chem. 2019 Aug 16;294(33):12547-12554. doi: 10.1074/jbc.RA119.007735. Epub 2019 Jul 2. J Biol Chem. 2019. PMID: 31266805 Free PMC article. - Raloxifene enhances spontaneous microaggregation of platelets through upregulation of p44/p42 MAP kinase: a case report.
Tokuda H, Harada A, Adachi S, Matsushima-Nishiwaki R, Natsume H, Minamitani C, Mizutani J, Otsuka T, Kozawa O. Tokuda H, et al. Osteoporos Int. 2010 Jan;21(1):189-93. doi: 10.1007/s00198-009-0927-9. Epub 2009 Apr 7. Osteoporos Int. 2010. PMID: 19350338 - Dengue Virus Entry and Replication Does Not Lead to Productive Infection in Platelets.
Kar M, Singla M, Chandele A, Kabra SK, Lodha R, Medigeshi GR. Kar M, et al. Open Forum Infect Dis. 2017 Mar 23;4(2):ofx051. doi: 10.1093/ofid/ofx051. eCollection 2017 Spring. Open Forum Infect Dis. 2017. PMID: 28491890 Free PMC article. - Distinct role of Pyk2 in mediating thromboxane generation downstream of both G12/13 and integrin αIIbβ3 in platelets.
Kim S, Cipolla L, Guidetti G, Okigaki M, Jin J, Torti M, Kunapuli SP. Kim S, et al. J Biol Chem. 2013 Jun 21;288(25):18194-203. doi: 10.1074/jbc.M113.461087. Epub 2013 May 2. J Biol Chem. 2013. PMID: 23640884 Free PMC article.
References
- Brass L. F., Manning D. R., Cichowski K., Abrams C. S. Signaling through G proteins in platelets: to the integrins and beyond. Thromb. Haemostasis. 1997;78:581–589. - PubMed
- Cattaneo M., Gachet C. ADP receptors and clinical bleeding disorders. Arterioscler., Thromb., Vasc. Biol. 1999;19:2281–2285. - PubMed
- Kahner B. N., Shankar H., Murugappan S., Prasad G. L., Kunapuli S. P. Nucleotide receptor signaling in platelets. J. Thromb. Haemostasis. 2006;4:2317–2326. - PubMed
- Trumel C., Payrastre B., Plantavid M., Hechler B., Viala C., Presek P., Martinson E. A., Cazenave J. P., Chap H., Gachet C. A key role of adenosine diphosphate in the irreversible platelet aggregation induced by the PAR1-activating peptide through the late activation of phosphoinositide 3-kinase. Blood. 1999;94:4156–4165. - PubMed
- Nieswandt B., Schulte V., Zywietz A., Gratacap M. P., Offermanns S. Costimulation of Gi- and G1213-mediated signaling pathways induces integrin αIIbβ3 activation in platelets. J. Biol. Chem. 2002;277:39493–39498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL060683/HL/NHLBI NIH HHS/United States
- T32 HL007777/HL/NHLBI NIH HHS/United States
- HL80444/HL/NHLBI NIH HHS/United States
- HL60683/HL/NHLBI NIH HHS/United States
- R01 HL080444/HL/NHLBI NIH HHS/United States
- T32 HL07777/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous