Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum - PubMed (original) (raw)
Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum
Kyle S Smith et al. J Neurosci. 2007.
Abstract
Mu-opioid stimulation of cubic millimeter hedonic hotspots in either the nucleus accumbens shell (NAc) or the ventral pallidum (VP) amplifies hedonic "liking" reactions to sweetness and appetitive "wanting" for food reward. How do these two NAc-VP hotspots interact? To probe their interaction and limbic circuit properties, we assessed whether opioid activation of one hotspot recruited the other hotspot (neurobiologically) and whether opioid hedonic and incentive motivational amplification by either opioid hotspot required permissive opioid coactivation in the other (behaviorally). We found that NAc and VP hotspots reciprocally modulated Fos expression in each other and that the two hotspots were needed together to enhance sucrose "liking" reactions, essentially cooperating within a single hedonic NAc-VP circuit. In contrast, the NAc hotspot dominated for opioid stimulation of eating and food intake ("wanting"), independent of VP activation. This pattern reveals differences between limbic opioid circuits that control reward "liking" and "wanting" functions.
Figures
Figure 1.
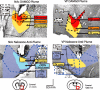
Experimental timeline and microinjection combinations. At time 0, rats were microinjected simultaneously in the NAc and VP with one of five combinations of drugs or vehicle centered in the hedonic hotspots shown in the top right (combinations depicted in “conditions” at bottom). For each combination, one drug (DAMGO or naloxone) or vehicle was administered bilaterally in NAc, and a different drug or vehicle was administered bilaterally in VP. A taste reactivity test of “liking” reactions to orally infused sucrose (1 min) was conducted at 30 min after microinjection (homologous tongue protrusion to sucrose shown in rat and human infant). Immediately after taste reactivity, spontaneous food intake was measured for the next 1.5 h. Fos plumes and distant Fos expression was assessed in separate rats, corresponding approximately to time of taste reactivity test and the onset of food intake test (Fos plume image, DAMGO). NAc and VP hotspots are modified from Peciña and Berridge (2005) and Smith and Berridge (2005).
Figure 2.
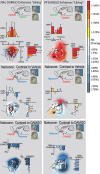
Examples of DAMGO Fos plumes and naloxone anti-plumes. Coronal sections show plume of local Fos elevation at tip of DAMGO microinjection (left top) and anti-plume of local Fos suppression at tip of naloxone microinjection in the NAc shell (left bottom). Equivalent DAMGO plume and naloxone anti-plume are shown for VP microinjections at right. Colors denote plume zones (elevation or suppression) mapped compared with spontaneous levels of Fos expression in unoperated normal tissue. Dashed lines denote plume zones mapped compared with Fos measured at corresponding points around vehicle microinjection sites (intensity of Fos change).
Figure 3.
DAMGO elevates Fos expression at distant sites, but naloxone suppresses distant Fos (sagittal view). Microinjection of DAMGO in the NAc shell elevates Fos expression in the VP and in anterior LH compared with vehicle (left top). Naloxone in NAc conversely suppresses Fos in VP and LH (left bottom). Colors denote regional magnitude of Fos change, and bar graphs show mean ± SE of percentage change compared with NAc–vehicle microinjection. Light gray boxes show Fos counting spots. Subregional comparison suggests that NAc opioid microinjections induced the greatest distant Fos change in posterior VP, containing VP hedonic hotspot. Distant Fos changes in NAc caused by VP microinjections of DAMGO or naloxone are shown at right. Subregional comparison suggests that greatest distant changes occurred in dorsorostral shell, containing NAc hedonic hotspot, after VP microinjections. Sagittal structure outlines modified from Paxinos and Watson (1998).
Figure 4.
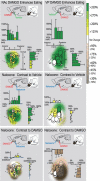
Fos plume map of hedonic “liking” effects of opioid activation (DAMGO) and blockade (naloxone). Sagittal views show inner Fos plumes as hexagons and outer plumes as surrounding shading (each unilateral site mapped separately is 2 symbols per rat). Symbol colors show percentage increase (yellow–red) or suppression (blue, stippling) of “liking” reactions elicited by sucrose after each DAMGO/naloxone combination [compared with either vehicle levels (top and middle) or “liking” enhancement after DAMGO alone (bottom)]. Bars along rostrocaudal and dorsoventral levels show intensity of drug effects within each 0.4-mm-wide level (mean ± SEM percentage of vehicle levels; a plume contributes to more than 1 bar if it straddles multiple levels). Left column shows “liking” effects of NAc–DAMGO and VP–naloxone combinations. DAMGO in NAc enhanced sucrose “liking” over vehicle levels (top left; contrast for DAMGO vs double-vehicle control). Addition of VP–naloxone to NAc–DAMGO blocks NAc–DAMGO enhancement over vehicle control levels (left middle; contrast to double-vehicle control). Addition of VP naloxone to NAc–DAMGO therefore reduced “liking” reactions to below levels produced by NAc–DAMGO alone (left bottom; contrast to DAMGO alone). Right column shows “liking” effects of VP–DAMGO and NAc–naloxone combinations. VP–DAMGO alone (NAc–vehicle) enhanced “liking” reactions to sucrose taste over double-vehicle levels (top right; contrast to double vehicle). Adding naloxone to NAc blocked VP–DAMGO ability to enhance “liking” reactions over vehicle (middle right; contrast to double vehicle) and therefore reduced “liking” to lower levels than after VP–DAMGO alone (bottom right; contrast to DAMGO alone). Sagittal structure outlines are modified from Paxinos and Watson (1998).
Figure 5.
Fos plume map of eating/intake effects of opioid activation (DAMGO) and blockade (naloxone). Organization is similar to Figure 4. Colors denote increase (green) or decrease (brown, stippling) in food intake caused by drug combinations. NAc–DAMGO stimulated eating and food intake when combined with VP–vehicle (top left; contrast to double vehicle) and even when combined with VP–naloxone (middle left; contrast to double vehicle). Adding naloxone in VP to NAc–DAMGO therefore failed to reduce intake much below levels after NAc–DAMGO alone (bottom left; contrast to DAMGO alone). However, although VP–DAMGO equally enhanced food intake (top right; contrast to double vehicle), adding NAc–naloxone almost completely blocked the intake increase normally caused by DAMGO in VP (middle right; contrast to double vehicle) and therefore reduced intake after the combination to below the high levels produced by VP–DAMGO alone (bottom right; contrast to DAMGO alone).
Figure 6.
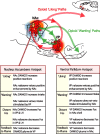
Summary of results and implications for NAc–VP limbic circuits of “liking” and “wanting.” Opioid hotspots in NAc and VP each can elevate “liking” reactions. Opioid enhancement of “liking” recruits the other hotspot and requires simultaneous participation by both, thus forming an interactive NAc–VP loop for amplification of taste “liking” reactions (red line). In contrast, for “wanting” stimulation, NAc can act independently of VP (but not vice versa), suggesting an alternative output route NAc opioid signals to eat (green line). The expanded inset is not to scale.
Similar articles
- An orexin hotspot in ventral pallidum amplifies hedonic 'liking' for sweetness.
Ho CY, Berridge KC. Ho CY, et al. Neuropsychopharmacology. 2013 Aug;38(9):1655-64. doi: 10.1038/npp.2013.62. Epub 2013 Mar 5. Neuropsychopharmacology. 2013. PMID: 23463152 Free PMC article. - Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness "liking" and "wanting".
Castro DC, Berridge KC. Castro DC, et al. J Neurosci. 2014 Mar 19;34(12):4239-50. doi: 10.1523/JNEUROSCI.4458-13.2014. J Neurosci. 2014. PMID: 24647944 Free PMC article. - Disentangling pleasure from incentive salience and learning signals in brain reward circuitry.
Smith KS, Berridge KC, Aldridge JW. Smith KS, et al. Proc Natl Acad Sci U S A. 2011 Jul 5;108(27):E255-64. doi: 10.1073/pnas.1101920108. Epub 2011 Jun 13. Proc Natl Acad Sci U S A. 2011. PMID: 21670308 Free PMC article. - Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry.
Castro DC, Cole SL, Berridge KC. Castro DC, et al. Front Syst Neurosci. 2015 Jun 15;9:90. doi: 10.3389/fnsys.2015.00090. eCollection 2015. Front Syst Neurosci. 2015. PMID: 26124708 Free PMC article. Review. - Ventral pallidum roles in reward and motivation.
Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Smith KS, et al. Behav Brain Res. 2009 Jan 23;196(2):155-67. doi: 10.1016/j.bbr.2008.09.038. Epub 2008 Oct 8. Behav Brain Res. 2009. PMID: 18955088 Free PMC article. Review.
Cited by
- Western Diet Consumption During Development: Setting the Stage for Neurocognitive Dysfunction.
Tsan L, Décarie-Spain L, Noble EE, Kanoski SE. Tsan L, et al. Front Neurosci. 2021 Feb 10;15:632312. doi: 10.3389/fnins.2021.632312. eCollection 2021. Front Neurosci. 2021. PMID: 33642988 Free PMC article. Review. - An orexin hotspot in ventral pallidum amplifies hedonic 'liking' for sweetness.
Ho CY, Berridge KC. Ho CY, et al. Neuropsychopharmacology. 2013 Aug;38(9):1655-64. doi: 10.1038/npp.2013.62. Epub 2013 Mar 5. Neuropsychopharmacology. 2013. PMID: 23463152 Free PMC article. - Social Reward and Empathy as Proximal Contributions to Altruism: The Camaraderie Effect.
Lahvis GP. Lahvis GP. Curr Top Behav Neurosci. 2017;30:127-157. doi: 10.1007/7854_2016_449. Curr Top Behav Neurosci. 2017. PMID: 27600591 Free PMC article. Review. - Evaluating the rewarding nature of social interactions in laboratory animals.
Trezza V, Campolongo P, Vanderschuren LJ. Trezza V, et al. Dev Cogn Neurosci. 2011 Oct;1(4):444-58. doi: 10.1016/j.dcn.2011.05.007. Epub 2011 Jun 2. Dev Cogn Neurosci. 2011. PMID: 22436566 Free PMC article. Review. - Unlucky punches: the vulnerability-stress model for the development of impulse control disorders in Parkinson's disease.
Theis H, Probst C, Fernagut PO, van Eimeren T. Theis H, et al. NPJ Parkinsons Dis. 2021 Dec 8;7(1):112. doi: 10.1038/s41531-021-00253-z. NPJ Parkinsons Dis. 2021. PMID: 34880241 Free PMC article. Review.
References
- Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–198. - PubMed
- Berridge KC. Pleasures of the brain. Brain Cogn. 2003;52:106–128. - PubMed
- Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. - PubMed
- Bodnar RJ. Endogenous opioids and feeding behavior: a 30-year historical perspective. Peptides. 2004;25:697–725. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DC010011/DC/NIDCD NIH HHS/United States
- MH63649/MH/NIMH NIH HHS/United States
- T32 DC000011/DC/NIDCD NIH HHS/United States
- DA015188/DA/NIDA NIH HHS/United States
- R01 MH063649/MH/NIMH NIH HHS/United States
- R01 DA015188/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials