A bile acid-like steroid modulates Caenorhabditis elegans lifespan through nuclear receptor signaling - PubMed (original) (raw)
A bile acid-like steroid modulates Caenorhabditis elegans lifespan through nuclear receptor signaling
Birgit Gerisch et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Broad aspects of Caenorhabditis elegans life history, including larval developmental timing, arrest at the dauer diapause, and longevity, are regulated by the nuclear receptor DAF-12. Endogenous DAF-12 ligands are 3-keto bile acid-like steroids, called dafachronic acids, which rescue larval defects of hormone-deficient mutants, such as daf-9/cytochrome P450 and daf-36/Rieske oxygenase, and activate DAF-12. Here we examined the effect of dafachronic acid on pathways controlling lifespan. Dafachronic acid supplementation shortened the lifespan of long-lived daf-9 mutants and abolished their stress resistance, indicating that the ligand is "proaging" in response to signals from the dauer pathways. However, the ligand extended the lifespan of germ-line ablated daf-9 and daf-36 mutants, showing that it is "antiaging" in the germ-line longevity pathway. Thus, dafachronic acid regulates C. elegans lifespan according to signaling state. These studies provide key evidence that bile acid-like steroids modulate aging in animals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
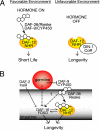
Fig. 1.
Dauer and germ-line longevity pathways. Δ4-dafachronic acid inhibits longevity in the dauer pathways but promotes it in the germ-line longevity pathway, showing a dependence on signaling context. Germ-line signaling is DAF-16/FOXO-dependent, whereas dauer signaling can be rendered DAF-16/FOXO-independent. Both contexts require DAF-12/nuclear hormone receptor. (A) Dauer longevity. Liganded DAF-12 promotes reproductive development and short life, whereas unliganded DAF-12 together with corepressor DIN-1 promote stress resistance and longevity. (B) Germ-line longevity. In the absence of signals from the germ line, liganded DAF-12, together with nuclear localized DAF-16/FOXO, promote longevity.
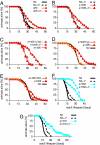
Fig. 2.
daf-9 longevity is hormone-dependent. The lifespan of adult animals was measured (in days) on OP50 bacterial lawns in the absence (solid line) or presence (dashed line) of added ligand (250 nM). Ligand was added during larval development and adulthood (Δ4) or during larval development only [Δ4(d)]. The experiments were performed at 15°C unless noted otherwise. Individual experiments are shown. Averages of mean and maximum lifespans are shown in Table 1. (A) Lifespan of wild-type N2 and daf-9(dh6). (B) Lifespan daf-9(e1406). (C) Lifespan of daf-9(e1406) when hormone was added during larval development only. (D) Lifespan of daf-9(e1406) in the presence of hypodermal-expressed wrt-1p::daf-9::gfp (hyp) during larval development. (E) Lifespan of daf-9(e1406) in the presence of daf-9::gfp expressed throughout life in the XXX neuroendocrine cells (XXX). (F) Lifespan of daf-2(e1368) at 22.5°C. (G) Lifespan of daf-2(e1370) at 22.5°C.
Fig. 3.
Lifespan in the germ-line longevity pathway is ligand-dependent. Strains were cultured at 25°C during larval development, and adult aging was carried out at 20°C in the absence (solid line) or presence (dashed line) of added ligand (250 nM). Pooled data of two experiments are shown. Averages of mean and maximum lifespans are shown in Table 1. (A) Lifespan of glp-1(e2141) and daf-36(k114) glp-1(e2141). (B) Lifespan of glp-1(e2141) and daf-9(rh50) glp-1(e2141). (C) Lifespan of glp-1(e2141) and daf-12(rh61rh411) glp-1(e2141). (D) Lifespan of wild-type N2 and daf-36(k114). (E) Lifespan of N2 and daf-9(rh50). (F) Lifespan of N2 and daf-12(rh61rh411).
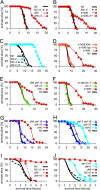
Fig. 4.
daf-9 stress resistance depends on ligand, daf-12(+) and din-1(+). (A–H) Thermotolerance. Young adult animals were shifted to 35°C, and survival was measured over time in the absence (solid line) or presence (dashed line) of added ligand (250 nM). Individual experiments are shown. Mean and maximum survival parameters are shown in
SI Table 3
. (I and J) Oxidative stress resistance. Young adult animals were shifted to plates containing 4.4–8.4 mM H2O2, and survival was measured over time in the absence (solid line) or presence (dashed line) of added ligand (250 nM). Individual experiments are shown. Mean and maximum survival parameters of individual experiments are shown in
SI Table 4
. (A) Thermotolerance of wild-type N2 and daf-9(dh6). (B) Thermotolerance of N2 and daf-9(e1406). (C) Thermotolerance of N2 and daf-2(e1370). (D) Thermotolerance of daf-9(e1406) in the presence of hypodermal-expressed wrt-1p::daf-9::gfp (hyp) during larval development and daf-9::gfp expressed throughout life in the XXX neuroendocrine cells (XXX). (E) Thermotolerance of daf-9(dh6) is abolished by daf-12(rh61rh411). (F) Thermotolerance of daf-9(e1406) is abolished by din-1(dh127). (G) Thermotolerance of daf-9(dh6) is reduced by daf-16(mgDf50). (H) Thermotolerance of daf-2(e1370) is reduced by daf-16(mg47). (I) Oxidative stress resistance of N2 and daf-9(dh6). (J) Oxidative stress resistance of N2 and daf-2(e1370).
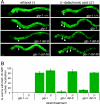
Fig. 5.
DAF-16::GFP localization to the intestinal nuclei in the germ-line longevity pathway is ligand-dependent. Animals were grown in the presence or absence of ligand at 25°C. DAF-16::GFP nuclear localization was scored on day 1 of adulthood. (A) Photomicrographs of DAF-16::GFP localization in the indicated genotypes, on ethanol vehicle (Left) or ligand (Right). Arrowheads indicate intestinal nuclei, arrows indicate cytoplasmic localization. (Scale bar: 10 μm.) (B) Bar graphs quantifying percentage of worms with DAF-16::GFP nuclear localization. Error bars indicate standard deviations from at least two independent trials (n > 160 animals).
Similar articles
- A steroid hormone that extends the lifespan of Caenorhabditis elegans.
Broué F, Liere P, Kenyon C, Baulieu EE. Broué F, et al. Aging Cell. 2007 Feb;6(1):87-94. doi: 10.1111/j.1474-9726.2006.00268.x. Aging Cell. 2007. PMID: 17266678 - Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans.
Motola DL, Cummins CL, Rottiers V, Sharma KK, Li T, Li Y, Suino-Powell K, Xu HE, Auchus RJ, Antebi A, Mangelsdorf DJ. Motola DL, et al. Cell. 2006 Mar 24;124(6):1209-23. doi: 10.1016/j.cell.2006.01.037. Epub 2006 Mar 9. Cell. 2006. PMID: 16529801 - Hormonal control of C. elegans dauer formation and life span by a Rieske-like oxygenase.
Rottiers V, Motola DL, Gerisch B, Cummins CL, Nishiwaki K, Mangelsdorf DJ, Antebi A. Rottiers V, et al. Dev Cell. 2006 Apr;10(4):473-82. doi: 10.1016/j.devcel.2006.02.008. Epub 2006 Mar 23. Dev Cell. 2006. PMID: 16563875 - Indicted: worms caught using steroids.
Beckstead RB, Thummel CS. Beckstead RB, et al. Cell. 2006 Mar 24;124(6):1137-40. doi: 10.1016/j.cell.2006.03.001. Cell. 2006. PMID: 16564008 Review. - Control of Caenorhabditis elegans life history by nuclear receptor signal transduction.
Rottiers V, Antebi A. Rottiers V, et al. Exp Gerontol. 2006 Oct;41(10):904-9. doi: 10.1016/j.exger.2006.06.062. Epub 2006 Sep 11. Exp Gerontol. 2006. PMID: 16963217 Review.
Cited by
- Sex-specific effects of the DAF-12 steroid receptor on aging in Caenorhabditis elegans.
McCulloch D, Gems D. McCulloch D, et al. Ann N Y Acad Sci. 2007 Nov;1119:253-9. doi: 10.1196/annals.1404.018. Ann N Y Acad Sci. 2007. PMID: 18056973 Free PMC article. - Aiweixin, a Traditional Uyghur Medicinal Formula, Extends the Lifespan of Caenorhabditis elegans.
Zhu B, Jo K, Yang P, Tohti J, Fei J, Abudukerim K. Zhu B, et al. Evid Based Complement Alternat Med. 2019 Jan 13;2019:3684601. doi: 10.1155/2019/3684601. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 30755775 Free PMC article. - New genes that extend Caenorhabditis elegans' lifespan in response to reproductive signals.
McCormick M, Chen K, Ramaswamy P, Kenyon C. McCormick M, et al. Aging Cell. 2012 Apr;11(2):192-202. doi: 10.1111/j.1474-9726.2011.00768.x. Epub 2011 Dec 28. Aging Cell. 2012. PMID: 22081913 Free PMC article. - The zinc matrix metalloproteinase ZMP-2 increases survival of Caenorhabditis elegans through interference with lipoprotein absorption.
Fischer M, Fitzenberger E, Kull R, Boll M, Wenzel U. Fischer M, et al. Genes Nutr. 2014 Jul;9(4):414. doi: 10.1007/s12263-014-0414-6. Epub 2014 Jun 24. Genes Nutr. 2014. PMID: 24957743 Free PMC article. - Empirical Validation of a Hypothesis of the Hormetic Selective Forces Driving the Evolution of Longevity Regulation Mechanisms.
Gomez-Perez A, Kyryakov P, Burstein MT, Asbah N, Noohi F, Iouk T, Titorenko VI. Gomez-Perez A, et al. Front Genet. 2016 Dec 6;7:216. doi: 10.3389/fgene.2016.00216. eCollection 2016. Front Genet. 2016. PMID: 27999589 Free PMC article.
References
- Kenyon C. Cell. 2005;120:449–460. - PubMed
- Hsin H, Kenyon C. Nature. 1999;399:362–366. - PubMed
- Jia K, Albert PS, Riddle DL. Development (Cambridge,UK) 2002;129:221–231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous