FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression - PubMed (original) (raw)
. 2007 Mar 13;104(11):4571-6.
doi: 10.1073/pnas.0700298104. Epub 2007 Mar 7.
Arabinda Samanta, Xiaomin Song, Kathryn T Iacono, Kathryn Bembas, Ran Tao, Samik Basu, James L Riley, Wayne W Hancock, Yuan Shen, Sandra J Saouaf, Mark I Greene
Affiliations
- PMID: 17360565
- PMCID: PMC1838642
- DOI: 10.1073/pnas.0700298104
FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression
Bin Li et al. Proc Natl Acad Sci U S A. 2007.
Abstract
The forkhead family protein FOXP3 acts as a repressor of transcription and is both an essential and sufficient regulator of the development and function of regulatory T cells. The molecular mechanism by which FOXP3-mediated transcriptional repression occurs remains unclear. Here, we report that transcriptional repression by FOXP3 involves a histone acetyltransferase-deacetylase complex that includes histone acetyltransferase TIP60 (Tat-interactive protein, 60 kDa) and class II histone deacetylases HDAC7 and HDAC9. The N-terminal 106-190 aa of FOXP3 are required for TIP60-FOXP3, HDAC7-FOXP3 association, as well as for the transcriptional repression of FOXP3 via its forkhead domain. FOXP3 can be acetylated in primary human regulatory T cells, and TIP60 promotes FOXP3 acetylation in vivo. Overexpression of TIP60 but not its histone acetyltransferase-deficient mutant promotes, whereas knockdown of endogenous TIP60 relieved, FOXP3-mediated transcriptional repression. A minimum FOXP3 ensemble containing native TIP60 and HDAC7 is necessary for IL-2 production regulation in T cells. Moreover, FOXP3 association with HDAC9 is antagonized by T cell stimulation and can be restored by the protein deacetylation inhibitor trichostatin A, indicating a complex dynamic aspect of T suppressor cell regulation. These findings identify a previously uncharacterized complex-based mechanism by which FOXP3 actively mediates transcriptional repression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
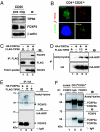
FOXP3 is acetylated, which is promoted by TIP60. (A) TIP60 expression in both human CD4+CD25+ T cells and CD4+CD25− T cells. FOXP3 and β-actin expression levels were also analyzed by immunoblotting with 221D and anti-β-actin antibodies. (B) Nuclear colocalization of endogenous FOXP3 with TIP60 in CD4+CD25+ T cells. Human CD4+CD25+ T cells were stimulated for 2 h with PMA/ionomycin, fixed, permeabilized, and stained by anti-FOXP3 hFOXY (eBioscience), in conjunction with rabbit anti-TIP60 (Upstate) as indicated. Cell nucleus was demonstrated by DAPI staining (blue panel). (C) FOXP3 associates with TIP60 in vivo. HEK 293T cells were cotransfected with expression plasmids for FLAG-TIP60, or HA-FOXP3a as indicated, immunoprecipitated with anti-FLAG M2, followed by Western blotting with anti-FOXP3 221D, or anti-FLAG M2. (D and E) TIP60 promotes FOXP3 acetylation. (D) HEK 293T cells were cotransfected with HA-FOXP3a and an increasing amount of FLAG-TIP60 as indicated, then immunoprecipitated either with acetylated-lysine Ac-K-103 (Upper) or with anti-HA F-7 probe (Lower), followed by Western blotting with HRP-HA. (E) HEK 293T cells were cotransfected with HA-FOXP3a and FLAG-TIP60 as indicated, then immunoprecipitated with anti-HA, followed by Western blotting either acetylated-lysine (Cell Signaling no. 9441; Upper) or HA-HRP (Lower). (F) FOXP3 is acetylated in human CD4+CD25+ T cells. Nuclear extracts from Jurkat E6.1 T cells and human CD4+CD25+ T cells were immunoprecipitated with anti-FOXP3 hFOXY, or control IgG, then analyzed with rabbit anti-acetyl-lysine (Upstate) (Upper) and reprobed with anti-FOXP3 221D (Lower).
Fig. 2.
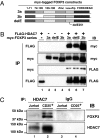
FOXP3 associates with HDAC7 in primary CD4+CD25+ T cells. (A) Schematic representation of the myc-tagged FOXP3 constructs used for detection of FOXP3-HDAC7 association. (B) FOXP3 associates with HDAC7. HEK 293T cells were cotransfected with FLAG-HDAC7, myc-FOXP3a (3a), or myc-FOXP3b (3b) as indicated. Cell lysates were either immunoprecipitated with anti-FLAG or anti-myc antibodies, then immunoblotted with indicated Abs. (C) Endogenous FOXP3 associates with HDAC7 in human CD4+CD25+ T cells. Nuclear extracts from Jurkat E6.1 T cells and human CD4+CD25+ T cells were immunoprecipitated with anti-HDAC7 C-18, or control IgG, then analyzed by Western blotting with anti-FOXP3 221D (Upper) and reprobed with anti-HDAC7 KG-17 (Lower).
Fig. 3.
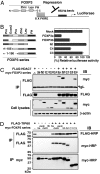
N-terminal 106–190 aa as the transcriptional repression domain of FOXP3 is essential for TIP60 and HDAC7 association. (A) Schematic representation of FOXP3 binding to 8x Forkhead-binding sites luciferase reporter construct (8x FK1TK-Luc) used in luciferase reporter assay. (B) Luciferase reporter assay using 8x FK1TK-Luc reporter. 293T cells were transfected with the control empty vector (mock), wild-type FOXP3a, FOXP3b, FOXP3 forkhead domain deletion (N1) expression vectors, or FOXP3 deletion mutant del C4 or delC3, plus 8x FK1TK-Luc luciferase reporter and control TK-Renilla luciferase vector as indicated, then analyzed by means of dual luciferase assay normalized with Renilla luciferase activity. Results are means of three separate experiments with SD. (C) HDAC7 associates with 3a, N1, and C4, but not C3 with an additional deletion of 106- to 190-aa region. 293T cells were transfected with a panel of myc-tagged FOXP3 expression vectors, combined with FLAG-HDAC7 as indicated, immunoprecipitated with anti-FLAG M2, then analyzed by Western blotting with indicated Abs. (D) TIP60 associates with 3a, 3b, delE, delK, N1, but not C3 with an additional deletion of 106- to 190-aa region. 293T cells were transfected with a panel of myc-tagged FOXP3 expression vectors, with or without FLAG-TIP60 as indicated, immunoprecipitated with either anti-FLAG or anti-myc mAb, then analyzed by Western blotting with indicated Ab.
Fig. 4.
FOXP3 mediates transcriptional repression via the forkhead domain as part of an ensemble with HDAC7 and TIP60. (A) Schematic representation of GAL4-FOXP3 binding to 5x GAL4-binding sites luciferase reporter construct (pG5_Luc_) used in luciferase reporter assay. (B) Overexpression of TIP60 promotes FOXP3-mediated transcriptional repression. 293T cells were transfected with the control pBIND empty vector (pBIND), pBIND-FOXP3a, pBIND-FOXP3a and pFLAG-TIP60 or, pBIND-FOXP3qa and the HAT-deficient TIP60 expressing construct (pFLAG-MUT-TIP60), plus pG5_Luc_ luciferase reporter and control MSV-β-Gal as indicated, then analyzed by means of luciferase assay normalized with β-Gal activity. Results are means of three separate experiments with SD. (C) Knockdown of endogenous TIP60 relieves FOXP3-mediated transcriptional repression. 293T cells were transfected with indicated vectors and cell lysates were analyzed by means of luciferase assay normalized with β-Gal activity. Results are means of 3 separated experiments with SD. The knockdown efficiency of TIP60 shRNA was evaluated by Western blotting with Rabbit anti-TIP60, and reprobed with anti-α-tubulin (Inset). (D) A role of FOXP3-TIP60-HDAC7 ensemble in the repression of IL-2 production. Transfected Jurkat E6.1 T cells with vectors as indicated were stimulated respectively with plate-bound TCR Vβ 8.1 plus soluble anti-CD28. IL-2 production in cultured medium was measured with IL-2 ELISA kit (eBioscience). The repression efficiency of the empty vector transfected sample was defined as zero, and the one with 10 μg each of FOXP3a, FOXP3b, TIP60, and HDAC7 plasmids transfected was defined as 100%. The result is the average ± standard error by mean of three independent experiments. (Inset) One representative result of three independent experiments showing the actual amount of IL-2 production after TCR plus CD28 stimulation in Jurkat T cells, which were cotransfected with either 10 μg each of FOXP3a, TIP60 and HDAC7 expressing plasmids, or equal amounts of empty vectors. Transfection of the HAT-deficient TIP60 or HDAC deficient HDAC7 species led to less inhibition of IL2 production.
Fig. 5.
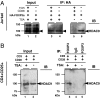
T cell stimulation antagonizes FOXP3 recruiting HDAC9. (A) HA-FOXP3a transfected Jurkat E6.1 T cells (10 × 106) were not stimulated, or stimulated with plate-bound TCR Vβ 8.1 plus soluble anti-CD28 for 4 h (with or without 400 nM TSA, indicated above lanes), lysed, and equal nuclear extracts were immunoprecipitated with anti-HA probe (F-7), then analyzed by immunoblotting with anti-HDAC9 H-45 (lanes 3–6). The input nuclear extracts of TSA treated cells, with or without stimulation, were also immunoblotted with anti-HDAC9 H-45 (lanes 1 and 2). (B) In vitro activated and expanded human CD4+CD25+ T cells were treated with or without 400 nM TSA and lysed, and equal nuclear extracts were immunoprecipitated with anti-FOXP3 mAb 221D or control IgG, then analyzed by immunoblotting with anti-HDAC9 H-45 (Right, lanes 3, 4, and 5). The input nuclear extracts of TSA treated or untreated cells were also immunoblotted with anti-HDAC9 H-45 (Left, lanes 1 and 2).
Similar articles
- Loss of epigenetic modification driven by the Foxp3 transcription factor leads to regulatory T cell insufficiency.
Bettini ML, Pan F, Bettini M, Finkelstein D, Rehg JE, Floess S, Bell BD, Ziegler SF, Huehn J, Pardoll DM, Vignali DA. Bettini ML, et al. Immunity. 2012 May 25;36(5):717-30. doi: 10.1016/j.immuni.2012.03.020. Epub 2012 May 10. Immunity. 2012. PMID: 22579476 Free PMC article. - Tip60 is a co-repressor for STAT3.
Xiao H, Chung J, Kao HY, Yang YC. Xiao H, et al. J Biol Chem. 2003 Mar 28;278(13):11197-204. doi: 10.1074/jbc.M210816200. Epub 2003 Jan 27. J Biol Chem. 2003. PMID: 12551922 - 60-kDa Tat-interactive protein (TIP60) positively regulates Th-inducing POK (ThPOK)-mediated repression of eomesodermin in human CD4+ T cells.
Li Y, Tsun A, Gao Z, Han Z, Gao Y, Li Z, Lin F, Wang Y, Wei G, Yao Z, Li B. Li Y, et al. J Biol Chem. 2013 May 31;288(22):15537-46. doi: 10.1074/jbc.M112.430207. Epub 2013 Apr 22. J Biol Chem. 2013. PMID: 23609452 Free PMC article. - FOXP3 actively represses transcription by recruiting the HAT/HDAC complex.
Li B, Greene MI. Li B, et al. Cell Cycle. 2007 Jun 15;6(12):1432-6. Epub 2007 May 10. Cell Cycle. 2007. PMID: 17592252 Review. - FOXP3 and its partners: structural and biochemical insights into the regulation of FOXP3 activity.
Zhou Z, Song X, Li B, Greene MI. Zhou Z, et al. Immunol Res. 2008;42(1-3):19-28. doi: 10.1007/s12026-008-8029-x. Immunol Res. 2008. PMID: 18626575 Review.
Cited by
- Comparative Genomics Reveals Key Gain-of-Function Events in Foxp3 during Regulatory T Cell Evolution.
Andersen KG, Nissen JK, Betz AG. Andersen KG, et al. Front Immunol. 2012 May 10;3:113. doi: 10.3389/fimmu.2012.00113. eCollection 2012. Front Immunol. 2012. PMID: 22590469 Free PMC article. - Searching for Peptide Inhibitors of T Regulatory Cell Activity by Targeting Specific Domains of FOXP3 Transcription Factor.
Lozano T, Casares N, Martil-Otal C, Anega B, Gorraiz M, Parker J, Ruiz M, Belsúe V, Pineda-Lucena A, Oyarzabal J, Lasarte JJ. Lozano T, et al. Biomedicines. 2021 Feb 17;9(2):197. doi: 10.3390/biomedicines9020197. Biomedicines. 2021. PMID: 33671179 Free PMC article. - Epigenetic regulation of human FOXP3+ Tregs: from homeostasis maintenance to pathogen defense.
Yue Y, Ren Y, Lu C, Li P, Zhang G. Yue Y, et al. Front Immunol. 2024 Jul 31;15:1444533. doi: 10.3389/fimmu.2024.1444533. eCollection 2024. Front Immunol. 2024. PMID: 39144146 Free PMC article. Review. - Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective.
Weaver CT, Hatton RD. Weaver CT, et al. Nat Rev Immunol. 2009 Dec;9(12):883-9. doi: 10.1038/nri2660. Nat Rev Immunol. 2009. PMID: 19935807 Review. - Mechanisms of human FoxP3+ Treg cell development and function in health and disease.
Attias M, Al-Aubodah T, Piccirillo CA. Attias M, et al. Clin Exp Immunol. 2019 Jul;197(1):36-51. doi: 10.1111/cei.13290. Epub 2019 Apr 1. Clin Exp Immunol. 2019. PMID: 30864147 Free PMC article. Review.
References
- Schwartz RH. Nat Immunol. 2005;6:327–330. - PubMed
- Schubert LA, Jeffery E, Zhang Y, Ramsdell F, Ziegler SF. J Biol Chem. 2001;276:37672–37679. - PubMed
- Hori S, Nomura T, Sakaguchi S. Science. 2003;299:1057–1061. - PubMed
- Fontenot JD, Gavin MA, Rudensky AY. Nat Immunol. 2003;4:330–336. - PubMed
- Khattri R, Cox T, Yasayko SA, Ramsdell F. Nat Immunol. 2003;4:337–342. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous