Sprouty-2 regulates oncogenic K-ras in lung development and tumorigenesis - PubMed (original) (raw)
. 2007 Mar 15;21(6):694-707.
doi: 10.1101/gad.1526207.
Alexander Meissner, James A Dowdle, Denise Crowley, Margaret Magendantz, Chensi Ouyang, Tiziana Parisi, Jayaraj Rajagopal, Leah J Blank, Roderick T Bronson, James R Stone, David A Tuveson, Rudolf Jaenisch, Tyler Jacks
Affiliations
- PMID: 17369402
- PMCID: PMC1820943
- DOI: 10.1101/gad.1526207
Sprouty-2 regulates oncogenic K-ras in lung development and tumorigenesis
Alice T Shaw et al. Genes Dev. 2007.
Erratum in
- Genes Dev. 2008 Jan 15;22(2):277
Abstract
Somatic activation of Ras occurs frequently in human cancers, including one-third of lung cancers. Activating Ras mutations also occur in the germline, leading to complex developmental syndromes. The precise mechanism by which Ras activation results in human disease is uncertain. Here we describe the phenotype of a mouse engineered to harbor a germline oncogenic K-rasG12D mutation. This mouse exhibits early embryonic lethality due to a placental trophoblast defect. Reconstitution with a wild-type placenta rescues the early lethality, but mutant embryos still succumb to cardiovascular and hematopoietic defects. In addition, mutant embryos demonstrate a profound defect in lung branching morphogenesis associated with striking up-regulation of the Ras/mitogen-activated protein kinase (MAPK) antagonist Sprouty-2 and abnormal localization of MAPK activity within the lung epithelium. This defect can be significantly suppressed by lentiviral short hairpin RNA (shRNA)-mediated knockdown of Sprouty-2 in vivo. Furthermore, in the context of K-rasG12D-mediated lung tumorigenesis, Sprouty-2 is also up-regulated and functions as a tumor suppressor to limit tumor number and overall tumor burden. These findings indicate that in the lung, Sprouty-2 plays a critical role in the regulation of oncogenic K-ras, and implicate counter-regulatory mechanisms in the pathogenesis of Ras-based disease.
Figures
Figure 1.
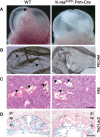
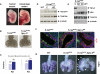
Extraembryonic phenotype of germline K-rasG12D mutants. (A) Intact visceral yolk sacs of wild-type (WT) and LSL-K-rasG12D;Prm-Cre embryos at E9.5. Large vitelline vessels are readily apparent in wild-type yolk sacs (arrow), but are absent from mutant yolk sacs. (B) Whole-mount staining of E9.5 yolk sacs with anti-PECAM antibody, a specific marker of differentiated endothelial cells. Arrows highlight yolk sac vitelline vessels. (C) H&E staining of E9.5 placentas. Fetal vessels containing nucleated embryonic erythroblasts (arrow) and maternal sinusoids containing adult enucleated red cells (arrowhead) are both present in the developing labyrinth of wild-type (WT) placentas. Mutant placentas contain maternal sinusoids (arrowhead), but lack fetal vessels. (D) LacZ staining of E9.5 placentas. Both wild-type (WT) and K-rasG12D embryos carry a Tie2-LacZ transgene to mark endothelial cells. Sections were counterstained with nuclear fast red. The dashed line demarcates the labyrinth layer of the placenta. (lb) Labyrinth; (sp) spongiotrophoblast layer; (gc) giant cell layer. Bars: C, 25 μm; D, 100 μm.
Figure 2.
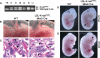
Rescue of early embryonic lethality by reconstitution of K-rasG12D embryos with wild-type placentas. (A) PCR of genomic DNA from various tissues of LSL-K-rasG12D;Mox2-Cre embryos showing recombination of the LSL element. The recombined K-rasG12D allele is readily distinguished from the wild-type (WT) K-ras allele by the presence of a single loxP site. Less recombination is detected in the yolk sac due to the extraembryonic endoderm present in this tissue. (Y) Yolk sac; (S) skin; (G) gut; (Lb) limb; (Lu) lungs; (B) brain; (Li) liver. (B) Intact visceral yolk sacs of wild-type (WT) and LSL-K-rasG12D;Mox2-Cre embryos at E12.5. A well-developed vascular network is present in both wild-type and mutant yolk sacs. (C) H&E staining of E12.5 placentas. Placentas supporting LSL-K-rasG12D;Mox2-Cre embryos show normal vascularization of the labyrinth. Arrows indicate examples of nucleated embryonic erythroblasts. Bar, 25 μm. (D) Wild-type (WT) and K-rasG12D embryos at E12.5 dissected free of the yolk sacs. Mutants appear morphologically normal, except for mild pallor of their fetal livers (arrow). (E) Wild-type (WT) and K-rasG12D embryos at E13.5. Mutants now exhibit pallor, generalized edema, and peripheral hemorrhages (arrowhead).
Figure 3.
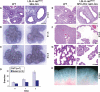
Disruption of lung epithelial branching morphogenesis by germline activation of K-ras. (A) H&E-stained transverse sections of E12.5 fetal lungs from wild-type (WT) and K-rasG12D embryos. (B,C) Lung branching patterns at E11.5 (B) and E12.5 (C). Shown are freshly isolated fetal lungs from wild-type (WT) and K-rasG12D embryos. (D) Quantitation of terminal branches. Lungs were isolated from E11.5 embryos and cultured in vitro for 2 d. Results are means ± SEM. (E–G) H&E-stained sections of fetal lungs from wild-type (WT) and LSL-K-rasG12D;SPC-rtTA;(tetO)7-Cre embryos at E13.5 (E), E16.5 (F), and E18.5 (G). At E13.5, doxycycline-treated triple mutants exhibit a severe defect in lung branching similar to that of germline K-rasG12D mutants shown in A. (H) Freshly isolated lungs from wild-type (WT) and LSL-K-rasG12D;SPC-rtTA;(tetO)7-Cre embryos at E18.5 showing persistence of the lung branching phenotype. Bars: A,E,F, 100 μm; G, 50 μm.
Figure 4.
Proliferation, cell survival, and differentiation in K-rasG12D fetal lungs. (A) Immunostaining of E12.5 fetal lungs for phospho-histone H3. (B) Immunostaining of E12.5 fetal lungs for BrdU. (C) Quantitation of phospho-histone H3-positive and BrdU-positive cells in the lung epithelium. The numbers of embryos analyzed are in parentheses. More than 1000 epithelial cells were counted per embryo. Results are means ± SEM. (D) Immunostaining of E12.5 fetal lungs for the apoptosis marker CC3. Neither wild-type (WT) nor K-rasG12D fetal lungs exhibit CC3-positive cells. (E,F) SP-C and β-tubulin immunofluorescence of E18.5 fetal lungs from wild-type (WT) and LSL-K-rasG12D;SPC-rtTA;(tetO)7-Cre embryos, both treated with doxycycline from E6.5 to E16.5. The numbers of embryos analyzed are in parentheses. (G,H) Immunohistochemical staining of E18.5 fetal lungs for CC10 and CGRP, markers of Clara cells and neuroendocrine cells, respectively. The numbers of embryos analyzed are in parentheses. In F_–_H, three step sections (100 μm) were examined per embryo. The bar graphs in F and G show the number of positive bronchi per lobe, with positive bronchi containing at least one positive cell. Bars: A,B,D,E, 50 μm; F, 100 μm; G,H, 25 μm.
Figure 5.
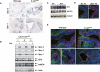
Induction of the Spry family of Ras/MAPK antagonists and aberrant MAPK activation in K-rasG12D fetal lungs. (A) In situ hybridization analysis of Spry expression in wild-type (WT) and K-rasG12D fetal lungs at E13.5. (B) Spry protein expression in three independently derived, LSL-K-rasG12D MEF lines, before and after treatment with Cre. (C) Immunoblotting of whole-cell lysates of E12.5 fetal lungs for phosphoErk. (D) Immunoblotting of wild-type (WT) fetal lungs demonstrating the specificity of pErk staining (red). The serial sections (10 μm apart) were photographed using the same exposure time. The actin cytoskeleton is stained in green with phalloidin; nuclei are stained blue with DAPI. (E) Immunohistochemical staining of E12.5 fetal lungs for phosphoErk. Wild-type (WT) lungs show a clear differential distribution of phosphoErk in the lung epithelium with sharp boundaries between areas of high and low Erk activity. In contrast, K-rasG12D lungs show uniform pErk expression in the lung epithelium with no sharp boundaries identifiable. Bars: D, 50 μm; E, 25 μm.
Figure 6.
Suppression of the lung branching defect of K-rasG12D embryos by lentiviral shRNA-mediated knockdown of Spry-2. (A) Gross appearance of control and knockdown mutant embryos. (B) Freshly isolated E12.5 lungs from LSL-K-rasG12D;Mox-Cre versus _LSL-K-rasG12D;Mox-Cre; Spry-2_−/− compound mutants. Lungs are photographed on a polyester membrane. (C) Quantitation of the rescue of lung branching by knockdown or knockout of Spry-2. Note that while Spry-2 knockdown or knockout increases the number of branches, the rescue is incomplete, as wild-type controls at E12.5 exhibit, on average, >20 terminal branches (see Fig. 3C). (D) Ras-GTP levels in fetal lungs derived from wild-type ES cells (WT), control infected LSL-K-rasG12D;SPC-rtTA;(tetO)7-Cre ES cells (C), and LSL-K-rasG12D;SPC-rtTA;(tetO)7-Cre ES cells expressing the Spry-2 shRNA (KD). All embryos were treated with doxycycline starting at E6.5. (E) Immunoblotting of fetal lungs for Spry-2 expression confirming efficient knockdown of Spry-2 in the fetal lungs of LSL-K-rasG12D;SPC-rtTA;(tetO)7-Cre;pSicoSpry-2 mutants. (F) Immunohistochemical staining of E12.5 fetal lung sections for phosphoErk. While K-rasG12D control lungs always show low-level, uniform MAPK activation throughout the epithelium, K-rasG12D;Spry-2KD lungs exhibit differential distribution of MAPK activity, with areas of intense activity juxtaposed with areas of low activity (arrow). Bar, 25 μm. (G) Whole-mount in situ hybridization of E12.5 fetal lungs. Compared with control K-ras mutants, K-ras mutants with the Spry-2 knockdown show increased Fgf10 expression, with numerous discrete areas of intense Fgf10 staining (arrows).
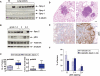
Figure 7.
Tumor suppression by Spry-2 in _K-rasG12D_-mediated lung tumorigenesis. (A) Induction of Spry proteins in mouse lung tumors induced by oncogenic activation of K-ras. Individual tumors were dissected from LA2 mice (see text) and compared with normal lung tissue (NI) from wild-type littermates. (B) Expression of Spry-2 and phosphoMAPK in mouse lung tumors of different histological grades. (C) Comparison of lung tumor number (left) and overall tumor burden (right) in LSL-K-rasG12D controls (n = 9) versus LSL-K-rasG12D;Spry-2Fl/Fl mice (n = 17). Tumor burden is measured as a ratio of total tumor area to total lung area. Boxes represent interquartile ranges (between the 25th and 75th quartiles). The total range and median are also shown. (D) H&E staining of lung tumors from LSL-K-rasG12D and LSL-K-rasG12D;Spry-2Fl/Fl mice. (E) Immunostaining of lung tumors for phosphoErk. A significant subset of both K-ras control and K-ras;Spry-2-null tumors express phosphoErk. In the absence of Spry-2, there appear to be more tumors with intense pErk staining. (F) Quantitation of pErk staining in K-ras control and K-ras;Spry-2-null lung tumors. pErk staining was scored based on the percentage of the tumor showing pErk expression: 0 (<10%), 1+ (10%–50%), and 2+ (>50%). Results are means ± SEM.
Similar articles
- Overexpression of Sprouty 2 in mouse lung epithelium inhibits urethane-induced tumorigenesis.
Minowada G, Miller YE. Minowada G, et al. Am J Respir Cell Mol Biol. 2009 Jan;40(1):31-7. doi: 10.1165/rcmb.2008-0147OC. Epub 2008 Jul 17. Am J Respir Cell Mol Biol. 2009. PMID: 18635814 Free PMC article. - Down-regulation of Sprouty2 in non-small cell lung cancer contributes to tumor malignancy via extracellular signal-regulated kinase pathway-dependent and -independent mechanisms.
Sutterlüty H, Mayer CE, Setinek U, Attems J, Ovtcharov S, Mikula M, Mikulits W, Micksche M, Berger W. Sutterlüty H, et al. Mol Cancer Res. 2007 May;5(5):509-20. doi: 10.1158/1541-7786.MCR-06-0273. Mol Cancer Res. 2007. PMID: 17510316 - Sprouty gain of function disrupts lens cellular processes and growth by restricting RTK signaling.
Shin EH, Zhao G, Wang Q, Lovicu FJ. Shin EH, et al. Dev Biol. 2015 Oct 15;406(2):129-46. doi: 10.1016/j.ydbio.2015.09.005. Epub 2015 Sep 13. Dev Biol. 2015. PMID: 26375880 - The Ras/MAPK pathway and hepatocarcinoma: pathogenesis and therapeutic implications.
Delire B, Stärkel P. Delire B, et al. Eur J Clin Invest. 2015 Jun;45(6):609-23. doi: 10.1111/eci.12441. Epub 2015 May 11. Eur J Clin Invest. 2015. PMID: 25832714 Review. - Reactive oxygen species and lung tumorigenesis by mutant K-ras: a working hypothesis.
Maciag A, Anderson LM. Maciag A, et al. Exp Lung Res. 2005 Jan-Feb;31(1):83-104. doi: 10.1080/01902140490495048. Exp Lung Res. 2005. PMID: 15765920 Review.
Cited by
- Control of mitotic spindle angle by the RAS-regulated ERK1/2 pathway determines lung tube shape.
Tang N, Marshall WF, McMahon M, Metzger RJ, Martin GR. Tang N, et al. Science. 2011 Jul 15;333(6040):342-345. doi: 10.1126/science.1204831. Science. 2011. PMID: 21764747 Free PMC article. - Overexpression of Sprouty 2 in mouse lung epithelium inhibits urethane-induced tumorigenesis.
Minowada G, Miller YE. Minowada G, et al. Am J Respir Cell Mol Biol. 2009 Jan;40(1):31-7. doi: 10.1165/rcmb.2008-0147OC. Epub 2008 Jul 17. Am J Respir Cell Mol Biol. 2009. PMID: 18635814 Free PMC article. - Expression of sprouty2 inhibits B-cell proliferation and is epigenetically silenced in mouse and human B-cell lymphomas.
Frank MJ, Dawson DW, Bensinger SJ, Hong JS, Knosp WM, Xu L, Balatoni CE, Allen EL, Shen RR, Bar-Sagi D, Martin GR, Teitell MA. Frank MJ, et al. Blood. 2009 Mar 12;113(11):2478-87. doi: 10.1182/blood-2008-05-156943. Epub 2009 Jan 15. Blood. 2009. PMID: 19147787 Free PMC article. - DA-Raf-dependent inhibition of the Ras-ERK signaling pathway in type 2 alveolar epithelial cells controls alveolar formation.
Watanabe-Takano H, Takano K, Sakamoto A, Matsumoto K, Tokuhisa T, Endo T, Hatano M. Watanabe-Takano H, et al. Proc Natl Acad Sci U S A. 2014 Jun 3;111(22):E2291-300. doi: 10.1073/pnas.1321574111. Epub 2014 May 19. Proc Natl Acad Sci U S A. 2014. PMID: 24843139 Free PMC article. - Report on the use of non-clinical studies in the regulatory evaluation of oncology drugs.
Hayakawa Y, Kawada M, Nishikawa H, Ochiya T, Saya H, Seimiya H, Yao R, Hayashi M, Kai C, Matsuda A, Naoe T, Ohtsu A, Okazaki T, Saji H, Sata M, Sugimura H, Sugiyama Y, Toi M, Irimura T. Hayakawa Y, et al. Cancer Sci. 2016 Feb;107(2):189-202. doi: 10.1111/cas.12857. Cancer Sci. 2016. PMID: 26919617 Free PMC article. Review.
References
- Aoki Y., Niihori T., Kawame H., Kurosawa K., Ohashi H., Tanaka Y., Filocamo M., Kato K., Suzuki Y., Kure S., Niihori T., Kawame H., Kurosawa K., Ohashi H., Tanaka Y., Filocamo M., Kato K., Suzuki Y., Kure S., Kawame H., Kurosawa K., Ohashi H., Tanaka Y., Filocamo M., Kato K., Suzuki Y., Kure S., Kurosawa K., Ohashi H., Tanaka Y., Filocamo M., Kato K., Suzuki Y., Kure S., Ohashi H., Tanaka Y., Filocamo M., Kato K., Suzuki Y., Kure S., Tanaka Y., Filocamo M., Kato K., Suzuki Y., Kure S., Filocamo M., Kato K., Suzuki Y., Kure S., Kato K., Suzuki Y., Kure S., Suzuki Y., Kure S., Kure S., et al. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat. Genet. 2005;37:1038–1040. - PubMed
- Bellusci S., Grindley J., Emoto H., Itoh N., Hogan B.L., Grindley J., Emoto H., Itoh N., Hogan B.L., Emoto H., Itoh N., Hogan B.L., Itoh N., Hogan B.L., Hogan B.L. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–4878. - PubMed
- Bos J.L. ras oncogenes in human cancer: A review. Cancer Res. 1989;49:4682–4689. - PubMed
- Braun B.S., Archard J.A., Van Ziffle J.A., Tuveson D.A., Jacks T.E., Shannon K., Archard J.A., Van Ziffle J.A., Tuveson D.A., Jacks T.E., Shannon K., Van Ziffle J.A., Tuveson D.A., Jacks T.E., Shannon K., Tuveson D.A., Jacks T.E., Shannon K., Jacks T.E., Shannon K., Shannon K. Somatic activation of a conditional KrasG12D allele causes ineffective erythropoiesis in vivo. Blood. 2006;108:2041–2044. - PMC - PubMed
- Cabernard C., Affolter M., Affolter M. Distinct roles for two receptor tyrosine kinases in epithelial branching morphogenesis in Drosophila. Dev. Cell. 2005;9:831–842. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5K08CA111634-2/CA/NCI NIH HHS/United States
- P30 CA014051/CA/NCI NIH HHS/United States
- U01 CA084306/CA/NCI NIH HHS/United States
- P30-CA14051/CA/NCI NIH HHS/United States
- K08 CA111634/CA/NCI NIH HHS/United States
- 5-U01-CA84306-06/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous