Missing-in-metastasis and IRSp53 deform PI(4,5)P2-rich membranes by an inverse BAR domain-like mechanism - PubMed (original) (raw)
Missing-in-metastasis and IRSp53 deform PI(4,5)P2-rich membranes by an inverse BAR domain-like mechanism
Pieta K Mattila et al. J Cell Biol. 2007.
Abstract
The actin cytoskeleton plays a fundamental role in various motile and morphogenetic processes involving membrane dynamics. We show that actin-binding proteins MIM (missing-in-metastasis) and IRSp53 directly bind PI(4,5)P(2)-rich membranes and deform them into tubular structures. This activity resides in the N-terminal IRSp53/MIM domain (IMD) of these proteins, which is structurally related to membrane-tubulating BAR (Bin/amphiphysin/Rvs) domains. We found that because of a difference in the geometry of the PI(4,5)P(2)-binding site, IMDs induce a membrane curvature opposite that of BAR domains and deform membranes by binding to the interior of the tubule. This explains why IMD proteins induce plasma membrane protrusions rather than invaginations. We also provide evidence that the membrane-deforming activity of IMDs, instead of the previously proposed F-actin-bundling or GTPase-binding activities, is critical for the induction of the filopodia/microspikes in cultured mammalian cells. Together, these data reveal that interplay between actin dynamics and a novel membrane-deformation activity promotes cell motility and morphogenesis.
Figures
Figure 1.
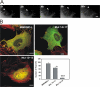
IMDs bind and tubulate PI(4,5)P2-rich membranes. (A) Native gel electrophoresis analysis was performed with MIM/IMD-L alone (without lipids) or with MIM/IMD-L mixed with fivefold molar excess of different phospholipids. PI(3,4)P2 and PI(4,5)P2 caused MIM/IMD-L to move faster toward the anode, indicating an increase in negative charge and a binding interaction. IP3, inositol(1,4,5) trisphosphate headgroup; PI, phosphatidylinositol; PA, phosphatidic acid; MIX, lipid mixture (cholesterol, lecithin, and lysolecithin); and CAR, cardiolipin. MIM/IMD-L without lipids was loaded to the first and last wells of the gels, respectively. (B) MIM/IMD-L and IRSp53/IMD cosedimented with PI(4,5)P2-rich (30%) large multilamellar vesicles. More than 50% of MIM/IMD-L was found in the pellet fraction (P) in samples containing PI(4,5)P2, whereas much weaker association is seen with vesicles without PI(4,5)P2. (C) EM analysis of multilamellar vesicles containing 30% of PI(4,5)P2 with and without MIM/IMD-L. Thin sections (60 nm) were visualized with transmission electron microscope. Images were taken with magnifications of 9,900 and 60,000. MIM/IMD-L clustered vesicles and deformed them into tubular network structures. Bars: (top) 1 μm; (bottom) 0.2 μm. (D) EM micrographs of 120-nm-thick sections of MIM/IMD-L deformed multilamellar (top) and unilamellar (bottom) vesicles containing 30% PI(4,5)P2. Bars, 0.2 μm. (E) 3D electron tomography analysis of MIM/IMD-L induced tubular network. (left) EM micrograph of a 250-nm-thick section; (right) corresponding model where tubules that were not connected with the surface of the membrane within the reconstructed volume were removed. Membrane tubules (diameter of 78 nm) penetrate the vesicular structure. Bar, 0.1 μm.
Figure 2.
Determination of PI(4,5)P2- and F-actin–binding sites of the IMD. (A) A list of mutants examined in this study. The PI(4,5)P2- and F-actin–binding properties of the mutants were determined by cosedimentation assays. The data obtained from F-actin–binding assays performed with four actin concentrations and from three independent PI(4,5)P2-binding assays are displayed (see Fig. S3, B and C, available at
http://www.jcb.org/cgi/content/full/jcb.200609176/DC1
, for the data). Affinities of mutant proteins as compared with wild-type IMD are indicated. (B) The residues mutated in this study are shown as ball and stick in the ribbon structure of MIM/IMD-L (Lee et al., 2007). Residues without detectable effects to PI(4,5)P2 binding are in green. Residues with moderate and strong defects in binding are in orange and in red, respectively. Substitution of Asp 143 (blue) by Ala resulted in an increase in PI(4,5)P2 binding. (C) Presentation of the F-actin–binding site of MIM/IMD-L with the same color coding.
Figure 3.
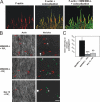
Compromised lipid binding weakens the ability of an IMD to induce dynamic filopodia. (A) Time-lapse images of a U2OS cell expressing GFP-tagged MIM/IMD-L. The black arrowhead shows the cell edge, and the white arrowhead indicates the tip of a growing filopodium. The filopodium extended with a rate of ∼1 μm/min. Bar, 1 μm. (B) Confocal images from U2OS cells overexpressing GFP-tagged wild-type and mutant forms of MIM/IMD-L. Mut12+15, which has severe defects in both F-actin and PI(4,5)P2 binding in vitro did not induce filopodia formation in cells. Mut14+17, which displayed moderate defects in PI(4,5)P2 binding but bound F-actin with wild-type affinity in vitro_,_ showed reduced filopodia formation compared with wild-type MIM/IMD-L. F-actin is in red. Bar, 10 μm. Quantification of the number of filopodia per cell is shown in the diagram. SEM values are indicated as error bars. Analysis (t test) of the number of filopodia shows results to be statistically significant (n = 20). ***, P < 0.001; *, P < 0.05.
Figure 4.
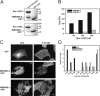
MIM/IMD-L localizes to the plasma membrane in filopodia and associates actin to membranes in vitro. (A) GFP–MIM/IMD-L localized to the plasma membrane and coated the F-actin bundles in filopodia of U2OS cell. 3D analysis derived from seven confocal planes shows F-actin in red (all panels), MIM/IMD in green (right), and colocalization in yellow (middle and right). Colocalization area (built by ImarisColoc software) is only a thin layer between actin and MIM/IMD and is almost invisible when the three channels are merged (right), as the MIM/IMD signal at the plasma membrane covers the interior of filopodia. Bar, 1 μm. (B) In vitro light microscopy assay. 1 μM F-actin (50% Alexa 568–labeled), 2.5 μM MIM/IMD-L, and 1.67 μM lipid vesicles (0/30% PI[4,5]P2) were mixed and applied on polyornithine-coated glass slides for imaging. In the presence of MIM/IMD-L and PI(4,5)P2, actin concentrated at the sites of vesicles, indicating MIM/IMD-L–associating actin and vesicles (top). MIM/IMD-L with vesicles without PI(4,5)P2 or MIM/IMD-L Mut15, which displays defects in actin and PI(4,5)P2 binding, induced much weaker colocalization between actin and vesicles. Red arrowheads indicate vesicles that show clear colocalization with actin, and green arrowheads indicate vesicles without colocalizing actin. Bar, 20 μm. (C) The intensity of the actin staining on the vesicles was quantified from 19–20 randomly selected vesicles. Error bars indicate SEM values. A t test was used to analyze p-values and showed statistically significant differences (***, P < 0.001; **, P < 0.01).
Figure 5.
IMDs display weak F-actin–bundling activity at physiological ionic strength. (A) Low-speed F-actin sedimentation assay, where actin bundles sedimented into the pellet fraction (P), whereas unbundled F-actin stayed in the supernatant (S). F-actin concentration was 2 μM, and MIM/IMD dimer concentrations were 0, 1.25, 2.5, and 5 μM. Human α-actinin was used as a control in dimer concentration of 1.25 μM. Note that although α-actinin efficiently cross-linked F-actin, neither MIM/IMD-L or -S induced detectable F-actin bundling in this assay. (B) Low-speed F-actin sedimentation assay repeated in buffers containing different concentrations (100, 75, 50, or 25 mM) of KCl. The actin-bundling activity of both MIM/IMD-L and IRSp53/IMD increased at lower KCl concentrations. (C) Quantification of F-actin–bundling activities of IMDs from three independent experiments at 100/50 mM KCl. Error bars indicate standard deviations. (D) DLS profiles of MIM/IMD-L at 100, 75, 50, or 25 mM KCl. The amount of aggregates >50 nm in hydrodynamic radius increased at lower KCl concentrations. The mean of R h distributions of two independent measurements is presented in the graphs.
Figure 6.
Interaction with small GTPase Rac is not required for IMD-induced filopodia formation. (A) The binding of MIM/IMD variants to Rac was determined by a GST pull-down assay under physiological ionic conditions. GST fusion of recombinant Rac (V12) or GST alone were coupled to glutathione–Sepharose beads and incubated with MIM/IMD splice variants. The beads were sedimented, washed three times, and loaded on SDS gels. MIM/IMD-S clearly bound Rac, whereas no binding of MIM/IMD-L was detected with this assay. (B) The binding of GST-Rac (V12) to MIM/IMD variants examined with a Biacore 2000. The histograms represent the equilibrium binding levels (Req) in resonance units that were obtained for each Rac concentration with binding of MIM/IMD-L or -S. (C) U2OS cells overexpressing GFP-tagged MIM/IMD-S or -L. Both MIM/IMD variants induced filopodia-like protrusions in cells in a similar manner. F-actin visualized with Alexa 568 phalloidin. Bar, 10 μm. (D) Quantification of filopodia number in GFP, GFP–MIM/IMD-L, and GFP–MIM/IMD-S transfected U2OS cells (n = 10). Between MIM/IMD splice variants no difference was seen, suggesting that Rac binding is not required for this activity.
Figure 7.
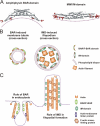
Schematic model for the mechanism of filopodia formation by IMDs. (A) Structures of amphiphysin BAR domain (left; Protein Data Bank ID: 1URU) and MIM/IMD (right; Protein Data Bank ID: 2D1L). The residues indicated in membrane binding in amphiphysin BAR (Itoh and De Camilli, 2006) and MIM/IMD (this study) are indicated in red. Purple dashed line indicates the proposed membrane interface. (B) Schematic model of membrane tubulation by BAR domain (left) and IMD (right). BAR domains form a coat outside of membrane tubule, whereas MIM/IMD curves the membrane in reversed orientation by binding to the inside of the tubule. (C) A side view of a BAR domain–induced endocytic invagination and an IMD-induced filopodium. Note that in addition to membrane-tubulating activities mediated by BAR and IMDs, both processes are also linked to actin dynamics.
Similar articles
- Molecular mechanisms of membrane deformation by I-BAR domain proteins.
Saarikangas J, Zhao H, Pykäläinen A, Laurinmäki P, Mattila PK, Kinnunen PK, Butcher SJ, Lappalainen P. Saarikangas J, et al. Curr Biol. 2009 Jan 27;19(2):95-107. doi: 10.1016/j.cub.2008.12.029. Epub 2009 Jan 15. Curr Biol. 2009. PMID: 19150238 - ABBA regulates plasma-membrane and actin dynamics to promote radial glia extension.
Saarikangas J, Hakanen J, Mattila PK, Grumet M, Salminen M, Lappalainen P. Saarikangas J, et al. J Cell Sci. 2008 May 1;121(Pt 9):1444-54. doi: 10.1242/jcs.027466. Epub 2008 Apr 14. J Cell Sci. 2008. PMID: 18413296 - I-BAR domains, IRSp53 and filopodium formation.
Ahmed S, Goh WI, Bu W. Ahmed S, et al. Semin Cell Dev Biol. 2010 Jun;21(4):350-6. doi: 10.1016/j.semcdb.2009.11.008. Epub 2009 Nov 11. Semin Cell Dev Biol. 2010. PMID: 19913105 Review. - Regulation of cell shape by Cdc42 is mediated by the synergic actin-bundling activity of the Eps8-IRSp53 complex.
Disanza A, Mantoani S, Hertzog M, Gerboth S, Frittoli E, Steffen A, Berhoerster K, Kreienkamp HJ, Milanesi F, Di Fiore PP, Ciliberto A, Stradal TE, Scita G. Disanza A, et al. Nat Cell Biol. 2006 Dec;8(12):1337-47. doi: 10.1038/ncb1502. Epub 2006 Nov 19. Nat Cell Biol. 2006. PMID: 17115031 - Subcellular membrane curvature mediated by the BAR domain superfamily proteins.
Suetsugu S, Toyooka K, Senju Y. Suetsugu S, et al. Semin Cell Dev Biol. 2010 Jun;21(4):340-9. doi: 10.1016/j.semcdb.2009.12.002. Epub 2009 Dec 4. Semin Cell Dev Biol. 2010. PMID: 19963073 Review.
Cited by
- Regulation of membrane-shape transitions induced by I-BAR domains.
Chen Z, Shi Z, Baumgart T. Chen Z, et al. Biophys J. 2015 Jul 21;109(2):298-307. doi: 10.1016/j.bpj.2015.06.010. Biophys J. 2015. PMID: 26200865 Free PMC article. - Phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) specifically induces membrane penetration and deformation by Bin/amphiphysin/Rvs (BAR) domains.
Yoon Y, Zhang X, Cho W. Yoon Y, et al. J Biol Chem. 2012 Oct 5;287(41):34078-90. doi: 10.1074/jbc.M112.372789. Epub 2012 Aug 11. J Biol Chem. 2012. PMID: 22888025 Free PMC article. - Molecular basis of the potent membrane-remodeling activity of the epsin 1 N-terminal homology domain.
Yoon Y, Tong J, Lee PJ, Albanese A, Bhardwaj N, Källberg M, Digman MA, Lu H, Gratton E, Shin YK, Cho W. Yoon Y, et al. J Biol Chem. 2010 Jan 1;285(1):531-40. doi: 10.1074/jbc.M109.068015. Epub 2009 Nov 1. J Biol Chem. 2010. PMID: 19880963 Free PMC article. - Role of the Scaffold Protein MIM in the Actin-Dependent Regulation of Epithelial Sodium Channels (ENaC).
Shuyskiy LS, Levchenko VV, Negulyaev YA, Staruschenko AV, Ilatovskaya DV. Shuyskiy LS, et al. Acta Naturae. 2018 Apr-Jun;10(2):97-103. Acta Naturae. 2018. PMID: 30116621 Free PMC article. - Pinkbar is an epithelial-specific BAR domain protein that generates planar membrane structures.
Pykäläinen A, Boczkowska M, Zhao H, Saarikangas J, Rebowski G, Jansen M, Hakanen J, Koskela EV, Peränen J, Vihinen H, Jokitalo E, Salminen M, Ikonen E, Dominguez R, Lappalainen P. Pykäläinen A, et al. Nat Struct Mol Biol. 2011 Jul 10;18(8):902-7. doi: 10.1038/nsmb.2079. Nat Struct Mol Biol. 2011. PMID: 21743456 Free PMC article.
References
- Bompard, G., S.J. Sharp, G. Freiss, and L.M. Machesky. 2005. Involvement of Rac in actin cytoskeleton rearrangements induced by MIM-B. J. Cell Sci. 118:5393–5403. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous