Mdm2 is required for inhibition of Cdk2 activity by p21, thereby contributing to p53-dependent cell cycle arrest - PubMed (original) (raw)
Mdm2 is required for inhibition of Cdk2 activity by p21, thereby contributing to p53-dependent cell cycle arrest
Luciana E Giono et al. Mol Cell Biol. 2007 Jun.
Abstract
p53 is extensively posttranslationally modified in response to various types of cellular stress. Such modifications have been implicated in the regulation of p53 protein levels as well as its DNA binding and transcriptional activities. Treatment of cells with doxorubicin causes phosphorylation and acetylation of p53, transcriptional upregulation of p21 and other target genes, and growth arrest. In contrast, downregulation of Mdm2 by a small interfering RNA (siRNA) approach led to increased levels of p53 lacking phosphorylation at serine 15 and acetylation at lysine 382. Levels of binding of p53 to the p21 promoter were comparable following treatment with doxorubicin or Mdm2 siRNA. Moreover, p53 was transcriptionally active and capable of inducing or repressing a variety of its target genes. Surprisingly, p53 upregulated by Mdm2 siRNA had no effect on cell cycle progression. Although comparable in level to that achieved by treatment with the p53 activators actinomycin D and nutlin-3, the increases in p53 and p21 after downregulation of Mdm2 were not sufficient to trigger cell cycle arrest. This version of p21 was capable of interacting with cyclin-dependent kinase 2 (Cdk2) but failed to inhibit its activity. Taken together, these results argue that Mdm2 is needed for full inhibition of Cdk2 activity by p21, thereby positively contributing to p53-dependent cell cycle arrest.
Figures
FIG. 1.
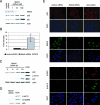
Downregulation of Mdm2 by siRNA causes p53 and p21 upregulation in the absence of DNA damage and posttranslational modifications. Mdm2 protein levels were downregulated in U2OS cells by use of siRNA oligonucleotides. (A and B) At 48 h after transfection, Mdm2, p53, p21, and actin levels in the cellular extracts were assayed by immunoblotting (A) and Mdm2 mRNA levels were quantitated by quantitative RT-PCR (B). (C and D) p53 stabilized by Mdm2 downregulation or 0.1 μg/ml doxorubicin (DOX) was analyzed for posttranslational modifications by immunoblotting. (E) At 48 h after transfection with control or Mdm2 siRNA or treatment with 0.1 μg/ml doxorubicin, U2OS cells were fixed and p53, Mdm2, γH2AX, and phosphorylated p53 (p-ser15) were localized by indirect immunofluorescence.
FIG. 2.
p53 upregulated by Mdm2 siRNA binds to the response elements in the p21 promoter both in vitro and in cells. U2OS cells were transfected with Mdm2 siRNA oligonucleotides or treated with 0.1 μg/ml doxorubicin (DOX) for 24 h. (A) Volumes of the different extracts containing similar amounts of p53 were used in oligonucleotide pull-down assays to measure binding to the p21 5′ biotinylated probe or a control probe (cont.). 1x and 2x represent two levels of whole cell extracts (wce), and “input” corresponds to 10% of those amounts. The presence of p53 in the precipitated complexes was detected by immunoblotting. (B) Cells were cross-linked, and extracts were subjected to ChIP analysis. Radioactive PCR was performed to detect the occupancy by p53 of the response elements in the p21 promoter. A nonrelated genomic region was amplified as a control. (C) p53 levels in the extracts used for ChIP were assayed by immunoblotting.
FIG. 3.
Upregulation of p53 by Mdm2 siRNA induces expression of p21 protein and mRNA to a level comparable to that seen with treatment with doxorubicin. (A) Clones of U2OS cells stably transfected with control (cont.) or p53 shRNA were transiently transfected with control or Mdm2 siRNA or treated with 0.1 μg/ml doxorubicin as indicated. Cell lysates were immunoblotted (left panel), and corresponding dishes were subjected to RT-PCR analysis (right panel). (B) HCT116 and U2OS cells were cotransfected with 200 ng of a luciferase reporter construct containing the p21 5′ response element, control or Mdm2 siRNA, and 5 ng of pRL-Renilla for internal control. At 24 h after transfection, cells were treated with 0.5 μg/ml doxorubicin when indicated. At 48 h after transfection, cells were lysed and assayed for luciferase and Renilla activities. The indicated values represent the averages of data obtained in three independent experiments, each performed in duplicate. Error bars represent standard errors of the means.
FIG. 4.
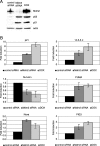
p53 upregulated by Mdm2 siRNA regulates transcription of various target genes. U2OS cells were transfected with control or Mdm2 siRNA or treated with doxorubicin (DOX) as indicated. At 48 h after treatment, cell lysates were immunoblotted (A) and mRNA levels from corresponding dishes were measured by quantitative RT-PCR (B).
FIG. 5.
p53 upregulated by Mdm2 siRNA fails to induce cell cycle arrest in U2OS cells. U2OS cells were transfected with control or Mdm2 siRNA or treated with doxorubicin (DOX) as indicated. At 24 and 48 h after transfection, cell lysates were immunoblotted (A) and corresponding dishes were pulsed with BrdU and subjected to staining and flow cytometry (B). The percentages of BrdU-positive cells are indicated at the top right corner of the panels.
FIG. 6.
p53 upregulated by Mdm2 siRNA fails to induce cell cycle arrest in HT1080, G361, and WI-38 cells. HT1080, G361, and WI-38 cells were transfected with control or Mdm2 siRNA or treated with 0.1 μg/ml doxorubicin (DOX) as indicated. At 24 and 48 h after transfection, cell lysates were immunoblotted, and at 24 h, corresponding dishes were pulsed with BrdU and subjected to staining and flow cytometry.
FIG. 7.
Induction of p53 and p21 to levels comparable to those seen with actinomycin D (Act.D) or nutlin-3 is not sufficient to trigger cell cycle arrest in U2OS cells following Mdm2 siRNA treatment. (A) Stable U2OS clones expressing control, p53, or p21 shRNA were treated with 5 nM actinomycin D or 2.5 μM nutlin-3. At 48 h after treatment, cell lysates were immunoblotted. untr., untreated. (B) Cells were treated as described above, and cell cycle profiles were analyzed by flow cytometry. The histogram shows the ratio of the percentage of cells in G1 phase to that of cells in S phase. (C) U2OS cells were transfected with control or Mdm2 siRNA oligonucleotides or were treated with actinomycin D or nutlin-3. At 48 h after treatment, cell lysates were immunoblotted. (D and E) Cells from corresponding dishes were stained with propidium iodide and analyzed by flow cytometry (D); the results are represented in panel E.
FIG. 8.
p21 upregulated following Mdm2 siRNA treatment localizes to the nucleus and binds to Cdk2 but does not inhibit Cdk2 kinase activity. (A) U2OS cells were grown on coverslips and transfected with control or Mdm2 siRNA or treated with doxorubicin as indicated. At 48 h after treatment, p21 was localized by indirect immunofluorescence. (B) U2OS cells were transfected with control or Mdm2 siRNA or treated with 5 nM actinomycin D (Act.D) or 2.5 μM nutlin-3. At 48 h after treatment, cell extracts were immunoprecipitated with anti-Cdk2 antibody. p21 and Cdk2 were detected in the immunoprecipitates (IP) by immunoblotting (WB). Cdk2 in cell extracts from corresponding dishes was immunoprecipitated and assayed for histone H1 kinase activity. (C) Protein levels in extracts used as described for panel B were assayed by immunoblotting. (D) U2OS cells were treated as described for panel B, and Mdm2, p21, pRb, and actin levels in the cellular extracts were assayed by immunoblotting. (E) p53−/− MEFs were infected with control or p53- or p21-encoding retroviruses. At 48 h after infection, cell lysates were immunoblotted. Cdk2 kinase activity from corresponding dishes was assayed as described, and the results are quantitated in panel F.
FIG. 9.
Cellular stress induces cell cycle arrest despite downregulation of Mdm2. (A and B) U2OS cells were transfected with the indicated siRNA oligonucleotides and treated with doxorubicin (DOX) (A) or actinomycin D (Act.D) (B) as described in Material and Methods. At 48 h after treatment, cell lysates were immunoblotted. (C) U2OS cells from corresponding dishes were stained with propidium iodide and analyzed by flow cytometry.
Similar articles
- The histone acetyltransferase PCAF regulates p21 transcription through stress-induced acetylation of histone H3.
Love IM, Sekaric P, Shi D, Grossman SR, Androphy EJ. Love IM, et al. Cell Cycle. 2012 Jul 1;11(13):2458-66. doi: 10.4161/cc.20864. Epub 2012 Jul 1. Cell Cycle. 2012. PMID: 22713239 Free PMC article. - Restoration of p53 pathway by nutlin-3 induces cell cycle arrest and apoptosis in human rhabdomyosarcoma cells.
Miyachi M, Kakazu N, Yagyu S, Katsumi Y, Tsubai-Shimizu S, Kikuchi K, Tsuchiya K, Iehara T, Hosoi H. Miyachi M, et al. Clin Cancer Res. 2009 Jun 15;15(12):4077-84. doi: 10.1158/1078-0432.CCR-08-2955. Epub 2009 Jun 9. Clin Cancer Res. 2009. PMID: 19509161 - Induction of apoptosis in non-small cell lung cancer by downregulation of MDM2 using pH-responsive PMPC-b-PDPA/siRNA complex nanoparticles.
Yu H, Zou Y, Jiang L, Yin Q, He X, Chen L, Zhang Z, Gu W, Li Y. Yu H, et al. Biomaterials. 2013 Apr;34(11):2738-47. doi: 10.1016/j.biomaterials.2012.12.042. Epub 2013 Jan 24. Biomaterials. 2013. PMID: 23352573 - Cell cycle regulation: p53-p21-RB signaling.
Engeland K. Engeland K. Cell Death Differ. 2022 May;29(5):946-960. doi: 10.1038/s41418-022-00988-z. Epub 2022 Mar 31. Cell Death Differ. 2022. PMID: 35361964 Free PMC article. Review. - To die or not to die: a HAT trick.
Tyteca S, Legube G, Trouche D. Tyteca S, et al. Mol Cell. 2006 Dec 28;24(6):807-8. doi: 10.1016/j.molcel.2006.12.005. Mol Cell. 2006. PMID: 17189182 Review.
Cited by
- p53-independent effects of Mdm2.
Bohlman S, Manfredi JJ. Bohlman S, et al. Subcell Biochem. 2014;85:235-46. doi: 10.1007/978-94-017-9211-0_13. Subcell Biochem. 2014. PMID: 25201198 Free PMC article. Review. - PAX3 in neuroblastoma: oncogenic potential, chemosensitivity and signalling pathways.
Fang WH, Wang Q, Li HM, Ahmed M, Kumar P, Kumar S. Fang WH, et al. J Cell Mol Med. 2014 Jan;18(1):38-48. doi: 10.1111/jcmm.12155. Epub 2013 Nov 4. J Cell Mol Med. 2014. PMID: 24188742 Free PMC article. - CDKN2A/B Homozygous Deletions in Astrocytomas: A Literature Review.
Yuile A, Satgunaseelan L, Wei JQ, Rodriguez M, Back M, Pavlakis N, Hudson A, Kastelan M, Wheeler HR, Lee A. Yuile A, et al. Curr Issues Mol Biol. 2023 Jun 22;45(7):5276-5292. doi: 10.3390/cimb45070335. Curr Issues Mol Biol. 2023. PMID: 37504251 Free PMC article. Review. - Mdm2 promotes Cdc25C protein degradation and delays cell cycle progression through the G2/M phase.
Giono LE, Resnick-Silverman L, Carvajal LA, St Clair S, Manfredi JJ. Giono LE, et al. Oncogene. 2017 Dec 7;36(49):6762-6773. doi: 10.1038/onc.2017.254. Epub 2017 Aug 14. Oncogene. 2017. PMID: 28806397 Free PMC article. - Tethering function of the caspase cleavage fragment of Golgi protein p115 promotes apoptosis via a p53-dependent pathway.
How PC, Shields D. How PC, et al. J Biol Chem. 2011 Mar 11;286(10):8565-8576. doi: 10.1074/jbc.M110.175174. Epub 2010 Dec 8. J Biol Chem. 2011. PMID: 21147777 Free PMC article.
References
- Appella, E., and C. W. Anderson. 2001. Post-translational modifications and activation of p53 by genotoxic stresses. Eur. J. Biochem. 268:2764-2772. - PubMed
- Boyd, M. T., N. Vlatkovic, and D. S. Haines. 2000. A novel cellular protein (MTBP) binds to MDM2 and induces a G1 arrest that is suppressed by MDM2. J. Biol. Chem. 275:31883-31890. - PubMed
- Brugarolas, J., C. Chandrasekaran, J. I. Gordon, D. Beach, T. Jacks, and G. J. Hannon. 1995. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377:552-557. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous