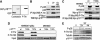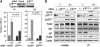A new p38 MAP kinase-regulated transcriptional coactivator that stimulates p53-dependent apoptosis - PubMed (original) (raw)
Comparative Study
. 2007 Apr 18;26(8):2115-26.
doi: 10.1038/sj.emboj.7601657. Epub 2007 Mar 22.
Affiliations
- PMID: 17380123
- PMCID: PMC1852783
- DOI: 10.1038/sj.emboj.7601657
Comparative Study
A new p38 MAP kinase-regulated transcriptional coactivator that stimulates p53-dependent apoptosis
Ana Cuadrado et al. EMBO J. 2007.
Abstract
The p38 mitogen-activated protein kinase (MAPK) signaling pathway plays an important role in stress-induced cell-fate decisions by orchestrating responses that go from cell-cycle arrest to apoptosis. We have identified a new p38 MAPK-regulated protein that we named p18(Hamlet), which becomes stabilized and accumulates in response to certain genotoxic stresses such as UV or cisplatin treatment. Overexpression of p18(Hamlet) is sufficient to induce apoptosis, whereas its downregulation reduces the apoptotic response to these DNA damage-inducing agents. We show that p18(Hamlet) interacts with p53 and stimulates the transcription of several proapoptotic p53 target genes such as PUMA and NOXA. This correlates with enhanced p18(Hamlet)-induced recruitment of p53 to the promoters. In proliferating cells, low steady-state levels of p18(Hamlet) are probably maintained by a p53-dependent negative feedback loop. Therefore, p18(Hamlet) is a new cell-fate regulator that links the p38 MAPK and p53 pathways and contributes to the establishment of p53-regulated stress responses.
Figures
Figure 1
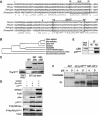
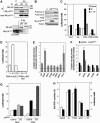
p18Hamlet is an evolutionarily conserved substrate of the p38α and p38β MAPKs. (A) Amino-acid sequence alignment of p18Hamlet proteins. Identical and similar residues are indicated by asterisks and two dots, respectively. The domains corresponding to the nuclear localization signal (NLS) and the zinc-finger HIT type I domain (Znf-HIT) are boxed. The arrowhead indicates a Thr residue that was selected to generate specific phospho antibodies. Sequence alignment was performed using the ClustalW program. (B) Phylogenetic tree of p18Hamlet proteins from Homo sapiens (NM_006349), Mus musculus (BC026751), Xenopus (NM_001017056), Drosophila (NP_608895), Danio rerio (AAH67648), Caenorhabditis elegans (NP_504477), Schizosaccharomyces pombe (CAB60106) and Saccharomyces cerevisiae (NP_013671). (C) GST pull-down assays were performed with the indicated GST-fused proteins and 35S-labelled p18Hamlet. (D) HEK-293 cells were transfected with 5 μg of Myc-p38α, MKK6DD and p18Hamlet, as indicated. Forty-eight hours after transfection, total cell lysates were prepared and analyzed by Western blotting, together with Myc IP. (E) HEK-293 cells were transfected with Myc-p38α, and 48 h after transfection, p38α was immunoprecipitated with Myc and HA (as a negative control) antibodies. (F) Kinase assays were performed using activated p38α and p38β MAPKs (200 ng) and GST, GST-p18Hamlet and GST-ATF-2 (1 μg) in the presence of 32P-γ-ATP. Coomassie staining shows the proteins used.
Figure 2
p38α phosphorylates several Thr residues in p18Hamlet. (A) GST-p18Hamlet or GST proteins (500 ng) were phosphorylated with p38α in vitro and then analyzed by Western blotting with phospho-Thr antibodies. (B) HEK-293 cells were transfected with Myc-p18Hamlet and 48 h later were treated with UV alone or in the presence of 10 μM SB203580. Three hours after irradiation, total cell lysates were prepared and analyzed by Western blotting, together with Myc immunoprecipitates. (C) HEK-293 cells were transfected with Myc-p18Hamlet wt and T103A either alone or together with MKK6DD, as indicated, and p18Hamlet phosphorylation was analyzed by Myc IP followed by Western blotting with phospho-Thr antibody. Total cell lysates were also analyzed by Western. (D) GST-p18Hamlet wt, T103A and T127A proteins were incubated with p38α and MKK6DD or with MKK6DD alone and then analyzed by Western blotting with both phospho-Thr103-p18Hamlet and generic phospho-Thr antibodies. (E) HeLa cells overexpressing p18Hamlet were UV irradiated, lysed at the indicated times after irradiation and analyzed by Western blotting with the phospho-Thr103-p18Hamlet antibody.
Figure 3
Accumulation of p18Hamlet protein in response to DNA damage-inducing agents. (A) MEFs and U2OS cells were treated with the proteasome inhibitor MG132 (25 μM) for 2 h and then lysed. Expression of endogenous p18Hamlet was analyzed by Western blotting. (B) U2OS cells were transfected with HA-ubiquitin and Myc-p18Hamlet, as indicated, and 16 h after transfection, were treated with MG132 (25 μM) for 5 h. Myc immunoprecipitates were analyzed by Western blotting using p18Hamlet and HA antibodies. (C) SK-Mel-103 and U2OS cells were treated with UV and cell lysates were analyzed by Western blotting using the indicated antibodies. (D) SK-Mel-103 cell lysates were prepared 3 h after UV treatment, either in the presence or absence of SB203580 (SB, 10 μM), and analyzed by Western blotting. (E) SK-Mel-103 cells were treated with cisplatin for the indicated times and p18Hamlet accumulation was analyzed by Western blotting. (F) U2OS cells were cotransfected with MKK6DD (600 ng), p18Hamlet (1 μg) and increasing amounts of p38α, as indicated. Twenty-four hours after transfection, lysates were prepared from both attached and floating cells (that express higher levels of p18Hamlet) and analyzed by Western blotting.
Figure 4
Stabilization of p18Hamlet protein in response to p38α activation. (A) U2OS cells were cotransfected with p18Hamlet (1 μg) and GFP (500 ng) and incubated with cycloheximide (CHX, 30 μg/ml) for the indicated times. Total cell lysates were analyzed by Western blotting. (B, C) U2OS cells were transfected with p18Hamlet, either alone or together with p38α and MKK6DD, and 24 h later were incubated with CHX for up to 4 h. Expression of p18Hamlet protein was determined by Western blotting. The blots corresponding to cells untreated or treated with CHX for 4 h, in the presence or absence of active p38α, are shown in (C). (D) Total RNAs were obtained from UV- or cisplatin-treated SK-Mel-103 cells and were analyzed by Northern blotting with a p18Hamlet probe. GAPDH was used to confirm equal RNA loading. (E) U2OS cells were transfected with 6 μg of p18Hamlet wt, T103A or 4 × T/A, as indicated. Twenty-four hours after transfection, cells were treated with CHX alone or in combination with MG132 for 4 h. Where indicated, cells were also UV irradiated 1 h before collection. A GFP expression vector (200 ng) was cotransfected to ensure equal efficiency of transfection.
Figure 5
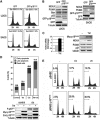
Induction of apoptosis by p18Hamlet overexpression. (A) U2OS and SAOS cells were transfected with GFP or GFP-p18Hamlet and analyzed by flow cytometry. The percentage of cells with a sub-G0/G1 DNA content in a representative experiment is shown. (B) U2OS and SAOS cells were transfected as indicated in (A) and the expression of the indicated proteins was analyzed by Western blotting. (C) U2OS cells expressing inducible p18Hamlet were treated with tetracycline for 24 h before RNA and protein extraction. Samples were analyzed by quantitative RT–PCRs (left) and Western blotting (right). (D) U2OS cells expressing tetracycline-inducible p18Hamlet were incubated with tet for 24 h and then treated with cisplatin for another 24 h before analyzing apoptosis by annexin V staining. Numbers on top of the bars indicate total percentage of early and late apoptotic events as well as dead cells. The lower panel shows the protein levels of overexpressed Myc-p18Hamlet, endogenous (End.) p18Hamlet (marked with an asterisk) and phospho-Ser15-p53. (E) Primary MEFs were infected with pBABE puro or p18Hamlet-expressing pBABE puro retroviruses. After puromycin selection, cells were treated for 24 h with cisplatin or UV as indicated, and the percentage of sub-G0/G1 cell population, as a measure of apoptosis levels, was analyzed by FACS.
Figure 6
p18Hamlet is required for apoptosis induction in response to DNA damage. (A) U2OS cells were transfected with p18Hamlet or control siRNAs and the levels of endogenous p18Hamlet were analyzed by Western blotting (upper). Forty-eight hours after transfection, cells were treated with UV or cisplatin for 24 h and apoptosis was quantified by measuring DNA fragmentation in a colorimetric assay. Means±standard deviations of three independent experiments are represented. Statistical significance was evaluated with the Student's _t_-test (_P_-values are shown). (B) U2OS cells were treated with siRNAs and UV or cisplatin as described in (A). Expression of the indicated proteins was detected by Western blotting.
Figure 7
p18Hamlet stimulates some p53-regulated genes. (A) Upper: U2OS cells with inducible p18Hamlet were treated with tetracycline (tet) for 24 h and total cell lysates were immunoprecipitated with p53 or control antibodies and then blotted with p18Hamlet antibodies. Lower_:_ total lysates of MG132-treated U2OS cells were immunoprecipitated and blotted as above to detect interaction between to endogenous p18Hamlet and p53. (B) U2OS cells expressing tet-inducible p18Hamlet were treated with tet or cisplatin and then analyzed by Western blotting. (C) U2OS cells were transfected with empty vector (control) and either p18Hamlet wt or p18Hamlet(1–117), together with reporter constructs containing different p53-responsive promoters upstream of the luciferase gene, as indicated. Luciferase activity was analyzed 16 h later and transfection efficiency was normalized to Renilla activity. Means±s.d. of three independent experiments are represented. (D) Transfections and luciferase assays were performed exactly as in (C) to test the wt and mutant PUMA 4 × BS2 reporters. (E) U2OS cells were transfected with the indicated reporter constructs. Twenty-four hours later, cells were treated with cisplatin and after 16 h, luciferase activity was measured and normalized to Renilla. (F) U2OS cells were transfected with p18Hamlet or control siRNAs and 24 h later cotransfected with the indicated luciferase reporters. Twenty-four hours after transfection, cells were incubated with cisplatin for 16 h and luciferase activity was measured. (G) U2OS cells were transfected with p18Hamlet or empty vector (control) in combination with Hdm2 and NOXA promoter reporters. Twenty four hours after transfection, cells were mock treated or treated with cisplatin and luciferase activity was measured 10 h later. Means±s.d. of three independent experiments are represented. (H) U2OS cells were transfected with GFP alone (control) or the indicated GFP-tagged p18Hamlet proteins and 48 h later the sub G0/G1 percentage in the fluorescent population was determined by FACS (white bars). U2OS cells were transfected with empty vector (control) or Myc-tagged wt and mutant p18Hamlet proteins, together with the PUMA 4 × BS2 reporter, and luciferase activity was measured 16 h later (black bars). Expression levels of Myc- and GFP-tagged p18Hamlet proteins are shown in Supplementary Figure 12.
Figure 8
Recruitment of p53 and p18Hamlet to p53-regulated promoters. (A) U2OS cells expressing tetracycline (tet)-inducible p18Hamlet were treated with tet for 24 h or UV irradiated for 8 h and then subjected to ChIP analysis. The DNA associated with the p53 immunoprecipitates was subjected to PCR with primers specific for the Hdm2, PUMA and NOXA promoters. (B) U2OS cells were treated with cisplatin for 6 h and then subjected to ChIP analysis using both p53 and p18Hamlet antibodies and NOXA primers. (C) p53 recruitment to NOXA promoter was analyzed by ChIP assay in U2OS cells 72 h after incubation with control and p18Hamlet siRNAs.
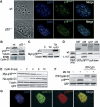
Figure 9
The p53 target gene cyclin G1 controls p18Hamlet protein levels under normal growing conditions. (A) MEFs (wt and p53−/−) were immunostained with p18Hamlet antibodies. Nuclear localization was confirmed by DAPI staining. (B) Expression of the indicated proteins was analyzed by Western blotting in wt and p53−/− MEFs. (C) U2OS cells with inducible p18Hamlet were treated with tetracycline (tet) and 24 h later were transfected with p53 and analyzed by Western blotting. (D) GST pull-down assay was performed by incubation of 35S-labelled p18Hamlet wt and 1–117 with GST and GST-fused p38α, cyclin G1 or p53, as indicated. (E) HEK-293 cells were cotransfected with Myc-p18Hamlet (5 μg) and increasing amounts of HA-cyclin G1 (0–10 μg). Twenty-four hours after transfection, the expression levels of the indicated proteins were analyzed by Western blotting using HA and Myc antibodies. Transfection efficiency was evaluated by cotransfection with GFP (500 ng). (F) HEK-293 cells were cotransfected with p18Hamlet (1 μg) and YFP-cyclin G1 (9 μg) and 16 h after transfection, cells were treated for 5 h with MG132. The expression levels of the indicated proteins were analyzed by Western blotting. (G) U2OS cells expressing tet-inducible p18Hamlet were transfected with HA-cyclin G1. Cellular localization was analyzed by immunostaining with HA and p18Hamlet antibodies. Colocalization areas are indicated in yellow (merge).
Similar articles
- p18(Hamlet) mediates different p53-dependent responses to DNA-damage inducing agents.
Lafarga V, Cuadrado A, Nebreda AR. Lafarga V, et al. Cell Cycle. 2007 Oct 1;6(19):2319-22. doi: 10.4161/cc.6.19.4741. Epub 2007 Jul 12. Cell Cycle. 2007. PMID: 17700068 - Puma, noxa, p53, and p63 differentially mediate stress pathway induced apoptosis.
Wang J, Thomas HR, Li Z, Yeo NCF, Scott HE, Dang N, Hossain MI, Andrabi SA, Parant JM. Wang J, et al. Cell Death Dis. 2021 Jun 30;12(7):659. doi: 10.1038/s41419-021-03902-6. Cell Death Dis. 2021. PMID: 34193827 Free PMC article. - Endoplasmic reticulum stress-induced apoptosis: multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53.
Li J, Lee B, Lee AS. Li J, et al. J Biol Chem. 2006 Mar 17;281(11):7260-70. doi: 10.1074/jbc.M509868200. Epub 2006 Jan 6. J Biol Chem. 2006. PMID: 16407291 - A systematic review of p53 regulation of oxidative stress in skeletal muscle.
Beyfuss K, Hood DA. Beyfuss K, et al. Redox Rep. 2018 Dec;23(1):100-117. doi: 10.1080/13510002.2017.1416773. Epub 2018 Jan 3. Redox Rep. 2018. PMID: 29298131 Free PMC article. Review. - Determination of cell fate by c-Abl activation in the response to DNA damage.
Kharbanda S, Yuan ZM, Weichselbaum R, Kufe D. Kharbanda S, et al. Oncogene. 1998 Dec 24;17(25):3309-18. doi: 10.1038/sj.onc.1202571. Oncogene. 1998. PMID: 9916993 Review.
Cited by
- Nuclear P38: Roles in Physiological and Pathological Processes and Regulation of Nuclear Translocation.
Maik-Rachline G, Lifshits L, Seger R. Maik-Rachline G, et al. Int J Mol Sci. 2020 Aug 24;21(17):6102. doi: 10.3390/ijms21176102. Int J Mol Sci. 2020. PMID: 32847129 Free PMC article. Review. - p16INK4A enhances the transcriptional and the apoptotic functions of p53 through DNA-dependent interaction.
Al-Khalaf HH, Nallar SC, Kalvakolanu DV, Aboussekhra A. Al-Khalaf HH, et al. Mol Carcinog. 2017 Jul;56(7):1687-1702. doi: 10.1002/mc.22627. Epub 2017 Mar 6. Mol Carcinog. 2017. PMID: 28218424 Free PMC article. - Myotubularin-related proteins 3 and 4 interact with polo-like kinase 1 and centrosomal protein of 55 kDa to ensure proper abscission.
St-Denis N, Gupta GD, Lin ZY, Gonzalez-Badillo B, Pelletier L, Gingras AC. St-Denis N, et al. Mol Cell Proteomics. 2015 Apr;14(4):946-60. doi: 10.1074/mcp.M114.046086. Epub 2015 Feb 6. Mol Cell Proteomics. 2015. PMID: 25659891 Free PMC article. - Physical and functional interactions between Drosophila homologue of Swc6/p18Hamlet subunit of the SWR1/SRCAP chromatin-remodeling complex with the DNA repair/transcription factor TFIIH.
Herrera-Cruz M, Cruz G, Valadez-Graham V, Fregoso-Lomas M, Villicaña C, Vázquez M, Reynaud E, Zurita M. Herrera-Cruz M, et al. J Biol Chem. 2012 Sep 28;287(40):33567-80. doi: 10.1074/jbc.M112.383505. Epub 2012 Aug 3. J Biol Chem. 2012. PMID: 22865882 Free PMC article. - The expanding universe of p53 targets.
Menendez D, Inga A, Resnick MA. Menendez D, et al. Nat Rev Cancer. 2009 Oct;9(10):724-37. doi: 10.1038/nrc2730. Nat Rev Cancer. 2009. PMID: 19776742 Review.
References
- Alonso G, Ambrosino C, Jones M, Nebreda AR (2000) Differential activation of p38 mitogen-activated protein kinase isoforms depending on signal strength. J Biol Chem 275: 40641–40648 - PubMed
- Barlev NA, Liu L, Chehab NH, Mansfield K, Harris KG, Halazonetis TD, Berger SL (2001) Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol Cell 8: 1243–1254 - PubMed
- Bergamaschi D, Samuels Y, O'Neil NJ, Trigiante G, Crook T, Hsieh JK, O'Connor DJ, Zhong S, Campargue I, Tomlinson ML, Kuwabara PE, Lu X (2003) iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human. Nat Genet 33: 162–167 - PubMed
- Bode AM, Dong Z (2004) Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer 4: 793–805 - PubMed
- Bulavin DV, Fornace AJ Jr (2004) p38 MAP kinase's emerging role as a tumor suppressor. Adv Cancer Res 92: 95–118 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous