Nippostrongylus brasiliensis: identification of intelectin-1 and -2 as Stat6-dependent genes expressed in lung and intestine during infection - PubMed (original) (raw)
Nippostrongylus brasiliensis: identification of intelectin-1 and -2 as Stat6-dependent genes expressed in lung and intestine during infection
David Voehringer et al. Exp Parasitol. 2007 Aug.
Abstract
Elimination of the helminth parasite Nippostrongylus brasiliensis from infected mice is mediated by IL-4 or IL-13 and dependent on the IL-4Ralpha chain and the transcription factor Stat6 in non-hematopoietic cells. However, it is not clear which Stat6-dependent effector molecules mediate worm expulsion. We identified intelectin-1 and -2 as Stat6-dependent genes that are induced during infection. Intelectins can bind galactofuranose, a sugar present only in microorganisms and might therefore serve as microbial pattern element. To analyze whether constitutive expression of intelectin-1 or -2 leads to accelerated pathogen clearance, transgenic mice were generated which express high levels of these genes selectively in the lung. Infection with N. brasiliensis or Mycobacterium tuberculosis did not result in accelerated pathogen clearance in transgenic as compared to wild-type mice. Further, no significant modulation of the immune response in lung or lymph nodes was observed. Thus, under these conditions, intelectins did not enhance pathogen clearance.
Figures
Figure 1
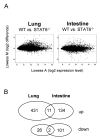
Microarray analysis of Stat6-dependent gene expression in lungs and small intestine after N. brasiliensis infection. A) Microarray analysis was performed 9 days after N. brasiliensis infection of BALB/c (WT) or Stat6-deficient mice (Stat6-/-) as described in Materials and Methods. Each dot corresponds to a single gene on the microarray. A-values on the x-axes indicate the expression levels on a log2 scale and M-values on the y-axes indicate the difference in expression levels between WT and Stat6-/- mice on a log2 scale. Horizontal lines indicate the cut-off for genes considered significantly up- or down-regulated in a Stat6-dependent fashion (M>1.5 or <-1.5, which corresponds to more than 2.8 fold up- or down-regulation, respectively). B) Venn diagram of genes that were up- or down-regulated by Stat6 in lung and/or intestine.
Figure 2
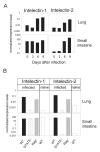
Quantitative RT-PCR for intelectin-1 and intelectin-2. A) Expression levels of intelectin-1 and intelectin-2 in lung and small intestine was analyzed on day 0, 3, 6 and 9 after N. brasiliensis infection of BALB/c mice by quantitative RT-PCR. B) Expression levels of intelectin-1 and intelectin-2 in lung and small intestine of naïve wild-type mice (WT naive) or day 9 _N. brasiliensis_-infected WT, Stat6-/- or Rag-/- mice were determined by quantitative RT-PCR. Data were normalized to HPRT. Error bars indicate standard deviations.
Figure 3
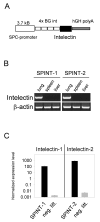
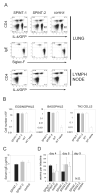
Expression of intelectin-1 and intelectin-2 in the lung of transgenic mice. A) Schematic representation of the expression vector used to generate intelectin-1 (SPINT-1) and intelectin-2 (SPINT-2) transgenic mice. B) RT-PCR from lung, spleen and liver of SPINT-1 and SPINT-2 transgenic mice with transgene-specific primers. C) Quantitative RT-PCR from lung of SPINT-1 or SPINT-2 mice and their negative littermates (neg. litt.) normalized to HPRT expression. Error bars indicate standard deviations.
Figure 4
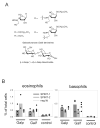
Administration of galactofuranose (Gal_f_) or galactopyranose (Gal_p_)containing–glycoconjugates. A) Chemical structures of the β-
D
-Gal_f_ glycosides and the β-
D
-Gal_p_-(1→6)-
D
-Gal_p_ disacharide used for intranasal administration. B) Recruitment of eosinophils and basophils to the lung was determined by flow cytometry 4 days after administration of pooled Gal_f_-glycosides or the Gal_p_-disacharide in SPINT-1 or SPINT-2 transgenic mice or their negative littermates. Untreated transgenic or wild-type mice served as control.
Figure 5
Analysis of the immune response of SPINT-1 and SPINT-2 transgenic mice after N. brasiliensis infection. A) Flow-cytometric analysis of lung and paratracheal lymph nodes of SPINT-1, SPINT-2 and 4get mice on day 9 after N. brasiliensis infection. SPINT mice were crossed to 4get mice to visualize IL-4-expressing cells ex vivo, as described in Materials and Methods. Dot-plots in the second row are gated on R1 (CD4-GFP+) from the dot-plots above and show the frequency of basophils (IgE+SiglecF-) and eosinophils (IgE-SiglecF+) within R1. B) Total number of eosinophils, basophils and Th2 cells in the lung on day 9 after infection. Uninfected wild-type mice were used as controls. C) Total serum IgE levels on day 9 after N. brasiliensis infection. D) The kinetics of worm expulsion was determined in SPINT-1, SPINT-2 and negative littermate control mice. Five mice per group were analyzed. Error bars indicate standard deviations.
Figure 6
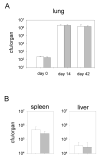
Infection of mice with aerosolized Mycobacterium tuberculosis.A) Intelectin transgenic mice (closed bars) or wild-type mice (open bars) were infected with M. tuberculosis as described in Materials and Methods. Colony forming units (cfu) in the lung were determined on day 0, 14 and 42 after infection. B) 42 days after infection cfu were determined in spleen and liver. 3-4 mice per group were analyzed. Error bars indicate standard deviations.
Similar articles
- Mucosal trapping and degradation of Nippostrongylus brasiliensis occurs in the absence of STAT6.
Van Panhuys N, Camberis M, Yamada M, Tegoshi T, Arizono N, Le Gros G. Van Panhuys N, et al. Parasitology. 2013 Jun;140(7):833-43. doi: 10.1017/S0031182012002260. Epub 2013 Feb 27. Parasitology. 2013. PMID: 23442551 - The roles of eotaxin and the STAT6 signalling pathway in eosinophil recruitment and host resistance to the nematodes Nippostrongylus brasiliensis and Heligmosomoides bakeri.
Knott ML, Matthaei KI, Foster PS, Dent LA. Knott ML, et al. Mol Immunol. 2009 Aug;46(13):2714-22. doi: 10.1016/j.molimm.2009.05.016. Epub 2009 Jun 16. Mol Immunol. 2009. PMID: 19535141 - IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis.
Urban JF Jr, Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, Finkelman FD. Urban JF Jr, et al. Immunity. 1998 Feb;8(2):255-64. doi: 10.1016/s1074-7613(00)80477-x. Immunity. 1998. PMID: 9492006 - Host protective roles of type 2 immunity: parasite killing and tissue repair, flip sides of the same coin.
Allen JE, Sutherland TE. Allen JE, et al. Semin Immunol. 2014 Aug;26(4):329-40. doi: 10.1016/j.smim.2014.06.003. Epub 2014 Jul 11. Semin Immunol. 2014. PMID: 25028340 Free PMC article. Review. - A comparative review of intelectins.
Chen L, Li J, Yang G. Chen L, et al. Scand J Immunol. 2020 Jul;92(1):e12882. doi: 10.1111/sji.12882. Epub 2020 Apr 14. Scand J Immunol. 2020. PMID: 32243627 Review.
Cited by
- Autophagy Protects against Colitis by the Maintenance of Normal Gut Microflora and Secretion of Mucus.
Tsuboi K, Nishitani M, Takakura A, Imai Y, Komatsu M, Kawashima H. Tsuboi K, et al. J Biol Chem. 2015 Aug 14;290(33):20511-26. doi: 10.1074/jbc.M114.632257. Epub 2015 Jul 6. J Biol Chem. 2015. PMID: 26149685 Free PMC article. - Gut microbiota alternation under the intestinal epithelium-specific knockout of mouse Piga gene.
Jangid A, Fukuda S, Seki M, Suzuki Y, Taylor TD, Ohno H, Prakash T. Jangid A, et al. Sci Rep. 2022 Jun 25;12(1):10812. doi: 10.1038/s41598-022-15150-5. Sci Rep. 2022. PMID: 35752737 Free PMC article. - Recognition of microbial glycans by human intelectin-1.
Wesener DA, Wangkanont K, McBride R, Song X, Kraft MB, Hodges HL, Zarling LC, Splain RA, Smith DF, Cummings RD, Paulson JC, Forest KT, Kiessling LL. Wesener DA, et al. Nat Struct Mol Biol. 2015 Aug;22(8):603-10. doi: 10.1038/nsmb.3053. Epub 2015 Jul 6. Nat Struct Mol Biol. 2015. PMID: 26148048 Free PMC article. - Stereoelectronic Effects Impact Glycan Recognition.
McMahon CM, Isabella CR, Windsor IW, Kosma P, Raines RT, Kiessling LL. McMahon CM, et al. J Am Chem Soc. 2020 Feb 5;142(5):2386-2395. doi: 10.1021/jacs.9b11699. Epub 2020 Jan 24. J Am Chem Soc. 2020. PMID: 31930911 Free PMC article. - Genome-wide transcriptomic analysis of intestinal tissue to assess the impact of nutrition and a secondary nematode challenge in lactating rats.
Athanasiadou S, Jones LA, Burgess ST, Kyriazakis I, Pemberton AD, Houdijk JG, Huntley JF. Athanasiadou S, et al. PLoS One. 2011;6(6):e20771. doi: 10.1371/journal.pone.0020771. Epub 2011 Jun 16. PLoS One. 2011. PMID: 21698235 Free PMC article.
References
- Daffe M, Brennan PJ, McNeil M. Predominant structural features of the cell wall arabinogalactan of Mycobacterium tuberculosis as revealed through characterization of oligoglycosyl alditol fragments by gas chromatography/mass spectrometry and by 1H and 13C NMR analyses. Journal of Biological Chemistry. 1990;265:6734–6743. - PubMed
- Finkelman FD, Shea-Donohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JF., Jr Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annual Review of Immunology. 1997;15:505–533. - PubMed
- Fujita T, Matsushita M, Endo Y. The lectin-complement pathway--its role in innate immunity and evolution. Immunological Reviews. 2004;198:185–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 HL056385/HL/NHLBI NIH HHS/United States
- P50 HL056385-090001/HL/NHLBI NIH HHS/United States
- HL56385/HL/NHLBI NIH HHS/United States
- AI30663/AI/NIAID NIH HHS/United States
- R01 AI030663-18A1/AI/NIAID NIH HHS/United States
- R01 AI030663/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous