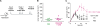Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development - PubMed (original) (raw)
Comparative Study
. 2007 May 14;204(5):1037-47.
doi: 10.1084/jem.20061120. Epub 2007 Apr 16.
Affiliations
- PMID: 17438063
- PMCID: PMC2118566
- DOI: 10.1084/jem.20061120
Comparative Study
Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development
Caroline Aspord et al. J Exp Med. 2007.
Abstract
We previously reported (Bell, D., P. Chomarat, D. Broyles, G. Netto, G.M. Harb, S. Lebecque, J. Valladeau, J. Davoust, K.A. Palucka, and J. Banchereau. 1999. J. Exp. Med. 190: 1417-1426) that breast cancer tumors are infiltrated with mature dendritic cells (DCs), which cluster with CD4(+) T cells. We now show that CD4(+) T cells infiltrating breast cancer tumors secrete type 1 (interferon gamma) as well as high levels of type 2 (interleukin [IL] 4 and IL-13) cytokines. Immunofluorescence staining of tissue sections revealed intense IL-13 staining on breast cancer cells. The expression of phosphorylated signal transducer and activator of transcription 6 in breast cancer cells suggests that IL-13 actually delivers signals to cancer cells. To determine the link between breast cancer, DCs, and CD4(+) T cells, we implanted human breast cancer cell lines in nonobese diabetic/LtSz-scid/scid beta2 microglobulin-deficient mice engrafted with human CD34(+) hematopoietic progenitor cells and autologous T cells. There, CD4(+) T cells promote early tumor development. This is dependent on DCs and can be partially prevented by administration of IL-13 antagonists. Thus, breast cancer targets DCs to facilitate its development.
Figures
Figure 1.
Breast cancer is infiltrated with CD4+ T cells secreting type 1 and type 2 cytokines. (A and B) Intracellular staining of IL-13 and IFN-γ on gated CD3+CD4+ T cells infiltrating breast cancer. Flow cytometry plots (A) and (B) represent samples from two different patients. To demonstrate specificity, anti–IL-13 mAb was pretreated with rhIL-13 before staining. (C) Representative flow cytometry analysis of CRTH2 expression by gated CD3+CD4+ T cells. In A–C, numbers indicate the percentage of cells positive for the specified marker. (D) Percentage of CRTH2+CD4+ T cells (ordinate) within breast cancer tumor or corresponding macroscopically not involved surrounding tissue (n = 6 patients). P = 0.03 using the paired t test.
Figure 2.
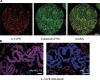
IL-13 staining on breast cancer cells. (A) Frozen breast cancer tumor sections were labeled with cytokeratin (green) and IL-13 (red). (B) Section from tumor obtained from a different patient. IL-13 staining of breast cancer cells can be inhibited by rhIL-13. Bars: (A) 20 μm; (B) 50 μm.
Figure 3.
Breast cancer cells express pSTAT6. Immunohistochemistry on paraffin-embedded tissue sections (A–C) and (D and E) represent tumors from two different patients. (A–C) A nest of breast cancer cells expressing cytokeratin (A) can also be stained with antibody recognizing STAT6 (B), as well as with an antibody recognizing pSTAT6 (C). (D and E) pSTAT6 staining is predominantly found on cancer nests (D) and can be blocked by a peptide used to generate the antibody (E). Bars, 100 μm.
Figure 4.
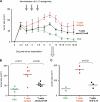
CD4+ T cells promote development of breast cancer tumors. (A) Experimental scheme. (B and C) 100 μl PBS (36 mice analyzed) and 10 × 106 autologous CD8+ T cells/100 μl PBS (21 mice analyzed) alone or together with 10 × 106 CD4+ T cells/100 μl PBS (55 mice analyzed) were transferred into Hs578T breast tumor–bearing humanized mice three times at days 3, 6, and 9 after tumor implantation. Kinetics of tumor development (mean ± SD; B, curves) and tumor size at day 12 (each dot represents one mouse; C) are shown. Horizontal bars represent the mean. (D) Experimental scheme. (E) CD4+ T cells generated as in A were purified from tumors and transferred into Hs578T tumor-bearing humanized mice. Tumor size at day 11 is shown (two experiments with four recipients per Humouse). Horizontal bars represent the mean.
Figure 5.
CD4+ T cells promote development of breast cancer tumors through DCs. (A) Experimental scheme. (B) 100 μl PBS or 10 × 106 CD4+ and CD8+ T cells/100 μl PBS were transferred into Hs578T tumor-bearing mice. The tumor size 12 d after tumor inoculation is shown for two experiments (nine mice per group). Horizontal bars represent the mean. (C) 100 μl PBS, and 106 DCs/100 μl PBS and 10 × 106 autologous CD4+ T cells/100 μl PBS, or both, were transferred into Hs578T tumor-bearing mice. Tumor growth was monitored (three mice per group; mean ± SD).
Figure 6.
CD4+ T cells isolated from tumors secrete IFN-γ as well as type 2 cytokines. 10 × 106 autologous CD4+ and CD8+ T cells /100 μl PBS were transferred into Hs578T breast tumor–bearing humanized mice at days 3, 6, and 9 after tumor inoculation. At day 15, T cells were purified from tumors and restimulated in vitro overnight with PMA/ionomycin. (A and B) Cytokine secretion was measured in the supernatant by Luminex (four humanized mice per group). Error bars represent the mean ± SEM. (C) Intracellular cytokine staining was also performed after restimulation in presence of Brefeldin A (B; two experiments with seven humanized mice). Numbers indicate the percentage of cells positive for the specified marker.
Figure 7.
Breast cancer instructs DCs to induce CD4+ T cells to secrete type 2 cytokines. (A) Experimental scheme. (B) Representative FACS analysis of breast cancer tumor cell suspension showing staining with HLA-DR (ordinate) and lineage (abscissa) mAbs. HLA-DR+Lin− DCs are sorted from tumors and draining lymph nodes and co-cultured for 5 d with allogeneic naive CD4+ T cells at a ratio 1:10. (C) Cytokine secretion to co-culture supernatants measured with multiplex bead analysis after overnight PMA/ionomycin restimulation. Each dot represents a separate Humouse tumor. Draining lymph nodes were pooled to obtain a sufficient number of cells for analysis. Horizontal bars represent the mean. (D) Intracellular cytokine expression by CD4+ T cells primed with DCs sorted from day 4 tumors and restimulated for 5 h with PMA/ionomycin in the presence of Brefeldin A. Dot plots are gated on CD3+CD4+ T cells (representative of n = 8 Humouse). Numbers indicate the percentage of cells positive for the specified marker. (E) IL-13 and IL-4 response in breast cancer, but not melanoma, environment. HLA-DR+Lin− DCs were sorted from lymph nodes draining Hs578T breast or Me275 melanoma tumors (day 4; lymph nodes were pooled from a total of 15 Humouse per group in four independent experiments). Horizontal bars represent the mean. P = 0.03 using the paired t test. (F) HLA-DR+Lin− DCs were sorted from tumors, draining lymph nodes, spleen, and BM of Hs578T breast cancer–bearing Humouse (day 30; n = 22 Humouse). Sorted DCs were co- cultured for 5 d with allogeneic naive CD4+ T cells at a ratio 1:10. Cytokine secretion was measured with multiplex bead analysis after overnight PMA/ionomycin restimulation. Box and whiskers data representation show median, range, and SE.
Figure 8.
CD4+ T cells promote tumor development via IL-13. 100 μl PBS or 10 × 106 autologous T cells/100 μl PBS were transferred into Hs578T breast tumor–bearing humanized mice at days 3 and 6 after tumor inoculation. Isotype or a mixture of anti–IL-13 antibody and rhIL-13Rα2/Fc chimera (100 μg per injection) were administrated at days 4, 6, and 8 after tumor implantation. (A) Kinetic of tumor size. Error bars represent the mean ± SEM. (B and C) Tumor size 13 d after tumor inoculation is shown (two experiments with six humanized mice per group). Data in C are from one of the experimental cohorts shown in B. Horizontal bars represent the mean.
Similar articles
- Splenic dendritic cells pulsed with Ixodes ricinus tick saliva prime naive CD4+T to induce Th2 cell differentiation in vitro and in vivo.
Mejri N, Brossard M. Mejri N, et al. Int Immunol. 2007 Apr;19(4):535-43. doi: 10.1093/intimm/dxm019. Epub 2007 Mar 6. Int Immunol. 2007. PMID: 17344202 - Transient depletion of CD4(+) T cells augments IL-21-based immunotherapy of disseminated neuroblastoma in syngeneic mice.
Croce M, Corrias MV, Orengo AM, Brizzolara A, Carlini B, Borghi M, Rigo V, Pistoia V, Ferrini S. Croce M, et al. Int J Cancer. 2010 Sep 1;127(5):1141-50. doi: 10.1002/ijc.25140. Int J Cancer. 2010. PMID: 20039320 - Flow cytometric analysis of circulating dendritic cell subsets and intracellular cytokine production in advanced breast cancer patients.
Ferrari S, Malugani F, Rovati B, Porta C, Riccardi A, Danova M. Ferrari S, et al. Oncol Rep. 2005 Jul;14(1):113-20. Oncol Rep. 2005. PMID: 15944777 - Investigational therapeutics targeting the IL-4/IL-13/STAT-6 pathway for the treatment of asthma.
Oh CK, Geba GP, Molfino N. Oh CK, et al. Eur Respir Rev. 2010 Mar;19(115):46-54. doi: 10.1183/09059180.00007609. Eur Respir Rev. 2010. PMID: 20956165 Free PMC article. Review.
Cited by
- Predictive model of gene expression regulating invasion and migration of M2 macrophages in breast cancer: clinical prognosis and therapeutic implications.
Jiang C, Luo J, Jiang X, Lv Y, Dou J. Jiang C, et al. Transl Cancer Res. 2024 Aug 31;13(8):4187-4204. doi: 10.21037/tcr-24-29. Epub 2024 Aug 21. Transl Cancer Res. 2024. PMID: 39262492 Free PMC article. - Immune Cell Migration to Cancer.
Ryan AT, Kim M, Lim K. Ryan AT, et al. Cells. 2024 May 16;13(10):844. doi: 10.3390/cells13100844. Cells. 2024. PMID: 38786066 Free PMC article. Review. - Upregulation of CCNB2 and a novel lncRNAs-related risk model predict prognosis in clear cell renal cell carcinoma.
Ren C, Wang Q, Xu Z, Pan Y, Wang S, Liu X. Ren C, et al. J Cancer Res Clin Oncol. 2024 Feb 1;150(2):64. doi: 10.1007/s00432-024-05611-x. J Cancer Res Clin Oncol. 2024. PMID: 38300330 Free PMC article. - Improving targeted small molecule drugs to overcome chemotherapy resistance.
Rismanbaf A. Rismanbaf A. Cancer Rep (Hoboken). 2024 Jan;7(1):e1945. doi: 10.1002/cnr2.1945. Epub 2023 Nov 22. Cancer Rep (Hoboken). 2024. PMID: 37994401 Free PMC article. Review. - Dual Functions of T Lymphocytes in Breast Carcinoma: From Immune Protection to Orchestrating Tumor Progression and Metastasis.
Zareinejad M, Mehdipour F, Roshan-Zamir M, Faghih Z, Ghaderi A. Zareinejad M, et al. Cancers (Basel). 2023 Sep 28;15(19):4771. doi: 10.3390/cancers15194771. Cancers (Basel). 2023. PMID: 37835465 Free PMC article. Review.
References
- Joyce, J.A. 2005. Therapeutic targeting of the tumor microenvironment. Cancer Cell. 7:513–520. - PubMed
- Steinman, R.M., D. Hawiger, and M.C. Nussenzweig. 2003. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21:685–711. - PubMed
- Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. - PubMed
- Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767–811. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA78846/CA/NCI NIH HHS/United States
- R01 CA085540/CA/NCI NIH HHS/United States
- CA85540/CA/NCI NIH HHS/United States
- U19 AI057234/AI/NIAID NIH HHS/United States
- R01 CA089440/CA/NCI NIH HHS/United States
- R01 CA078846/CA/NCI NIH HHS/United States
- R01 CA89440/CA/NCI NIH HHS/United States
- R21 AI056001/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials