Expression of lymphotoxin-alphabeta on antigen-specific T cells is required for DC function - PubMed (original) (raw)
Comparative Study
. 2007 May 14;204(5):1071-81.
doi: 10.1084/jem.20061968. Epub 2007 Apr 23.
Affiliations
- PMID: 17452522
- PMCID: PMC2118582
- DOI: 10.1084/jem.20061968
Comparative Study
Expression of lymphotoxin-alphabeta on antigen-specific T cells is required for DC function
Leslie E Summers-DeLuca et al. J Exp Med. 2007.
Abstract
During an immune response, activated antigen (Ag)-specific T cells condition dendritic cells (DCs) to enhance DC function and survival within the inflamed draining lymph node (LN). It has been difficult to ascertain the role of the tumor necrosis factor (TNF) superfamily member lymphotoxin-alphabeta (LTalphabeta) in this process because signaling through the LTbeta-receptor (LTbetaR) controls multiple aspects of lymphoid tissue organization. To resolve this, we have used an in vivo system where the expression of TNF family ligands is manipulated only on the Ag-specific T cells that interact with and condition Ag-bearing DCs. We report that LTalphabeta is a critical participant required for optimal DC function, independent of its described role in maintaining lymphoid tissue organization. In the absence of LTalphabeta or CD40L on Ag-specific T cells, DC dysfunction could be rescued in vivo via CD40 or LTbetaR stimulation, respectively, suggesting that these two pathways cooperate for optimal DC conditioning.
Figures
Figure 1.
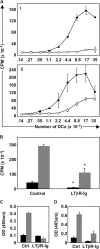
Signals mediated by the LTβR control DC function. (A) The stimulating capacity of DCs from OVA-immunized mice treated with either control Ig or LTβR-Ig was evaluated ex vivo. Draining LN DCs were harvested from each huIgG control-treated (filled circles) and LTβR-Ig–treated (empty circles) mice and plated with naive OTII responder T cells either without (i) or with (ii) exogenous OVA peptide. (B) The experiment was repeated as in A, but using naive OTI responder T cells. Black bars, wells with 5,000 DCs; gray bars, 20,000 DCs. IFNγ secretion by responder OTII cells (C) or OTI cells (D) in the absence of added OVA peptide was quantified by measuring IFNγ levels in cultured supernatants by ELISA (gray bars, 20,000 input DCs; black bars, 140 input DCs). These experiments were performed three times on five mice per group with similar results.
Figure 2.
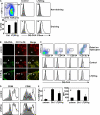
Expression of some DC maturation markers, but not antigen processing by DCs, is dependent on LTβR signaling. (A) Control or LTβR-Ig–treated mice received OTII T cells, were immunized with DQ-OVA+LPS, and DCs from draining (v and vi) and nondraining (ii and iii) LNs were gated as indicated (i) and analyzed for their processed DQ-OVA content by evaluating the fluorescence emitted at 515 nm. Gray-filled histograms are DCs from unimmunized mice, and empty histograms are DCs from DQ-OVA–immunized mice. Results are summarized in iv as a frequency of DCs that have acquired DQ-OVA, with gray bars representing unimmunized mice and black bars representing DQ-OVA–immunized mice. (B) Cryosections of popliteal LNs from either control-treated unimmunized or control versus LTβR-Ig–treated immunized mice were visualized with a 20× objective (Leica) for localization of proteolytically digested DQ-OVA and colocalization with CD11c+ DCs. B-cell follicles and the subcapsular sinus (SCS) are identified. (C) DC subsets were analyzed for DQ-OVA content by first eliminating TCR+CD19+ lymphocytes from analysis and gating on CD11c+ cells, as in A (i). Note that the CD11c Ab used in analyzing the CD11b+ DC subset was conjugated to a different fluorochrome than for the other FACS cocktails. (D) CD11c+ DQ-OVA+ DC were analyzed for the expression of CD80 and CD86. Percentage of CD86-high DC was tabulated, as well as the mean fluorescence intensity (MFI) (E). The flow cytometry experiment was performed twice on a total of 10 mice per group. The immunohistochemistry images are representative examples of six different mice per group. Bar, 100 μm.
Figure 3.
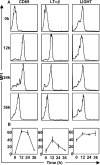
Expression of LTβR ligands on OTII CD4+ T cells in response to OVA antigen. Purified OTII T cells were adoptively transferred into C57BL/6 mice, which were then immunized with OVA. Thy1.1+ OTII T cells in the draining LNs were analyzed for the expression of CD69, LTαβ, and LIGHT. The expression of LTαβ and LIGHT on WT OTII T cells is demonstrated with representative histograms from five mice (A), and a kinetic analysis of expression of CD69, LTαβ, and LIGHT was generated (B). The experiment was performed twice with similar results. Expression on respective knockout T cells was insignificant over background (not depicted).
Figure 4.
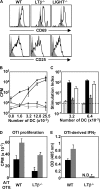
Function of DCs conditioned by adoptively transferred WT-, LTβ−/−-, or LIGHT−/−-OTII T cells. (A) C57BL/6 mice received WT-, LTβ−/−-, or LIGHT−/−-OTII T cells and were immunized, and LN cell suspensions were gated on CD4 and Thy1.1 and analyzed for CD69 and CD25 at 36 h after immunization, with filled histograms representing Thy1.1 T cells transferred into WT-unimmunized mice. (B) C57BL/6 mice received OVA-specific WT- (filled squares, filled circles), LTβ−/−- (empty circles), or LIGHT−/−-OTII T cells (empty triangles), or were immunized or left unimmunized (filled circles). At 36 h after immunization, draining LN DCs were plated with OTII responder T cells and incubated at 37°C for 72 h. (C) Proliferation results from B are represented as a SI. CPM derived from OTII T cells cocultured with DCs was divided by CPM derived from OTII T cells cocultured with the same number of DC-depleted cells at the same cell input number for each individual group (internally controlled). Groups are responder OTII T cells stimulated by DCs conditioned in vivo by WT- (black bars), LTβ−/−- (gray bars), or LIGHT−/−-OTII T cells (open bars) and compared with DCs from unimmunized mice that received WT-OTII T cells (speckled bars). (D) A similar experiment was performed using DCs conditioned by WT-OTII versus LTβ−/−-OTII to stimulate naive responder OTI T cells using 15,000 or 30,000 DCs per well (black and gray bars, respectively). (E) IFNγ secretion by OTI CD8+ T cells from was evaluated by ELISA using 15,000 or 30,000 DCs per well (black and gray bars, respectively). OTII responder experiments were performed four times using DCs pooled from seven individual animals. OTI responder experiments were performed two times using DCs pooled from seven individual animals.
Figure 5.
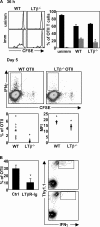
LTαβ–LTβR interactions are required for optimal CD4 priming in vivo. (A) C57BL/6 mice received CFSE-labeled, OVA-specific WT- or LTβ−/−-OTII T cells, were immunized with OVA+LPS, or were left unimmunized. At 36 h after immunization, OTII CFSE content was assessed, comparing baseline CFSE content for each individual genotype. At day 5 after immunization, secretion of IFNγ from OTII T cells was assessed by intracellular FACS. (B) C57BL/6 mice received OVA-specific WT-OTII T cells, were treated with huIgG or LTβR-Ig, and were immunized with OVA+LPS. At day 7, the frequency of IFNγ+ OTII T cells was assessed. The experiments were performed twice with three to five mice per group in each experiment.
Figure 6.
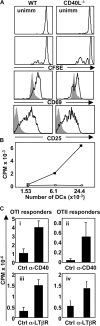
Expression of CD40L on OTII T cells is required for DC function, and agonistic anti-CD40 or -LTβR treatment can rescue DCs conditioned by LTβ−/−-OTII or CD40L−/−-OTII T cells, respectively. (A) C57BL/6 mice received CFSE-labeled WT- or CD40L−/−-OTII T cells, were immunized with OVA-LPS s.c., or were left unimmunized. CFSE content and the expression of CD69 and CD25 were measured by FACS at 36 h after immunization. (B) Draining LN DCs from mice immunized as in A were plated with OTII responder T cells and incubated at 37°C for 72 h. Filled circles, WT-OTII T cells; empty circles, CD40L−/−-OTII T cells. (C) C57BL/6 mice received LTβ−/−- (i and ii) or CD40L−/−-OTII T cells (iii and iv), were immunized with OVA-LPS s.c., and were treated with the indicated agonistic Ab or control Ab. Purified draining LN DCs (20,000 DCs for i and ii; 10,000 DCs for iii and iv) were plated with OTI and OTII responder T cells and incubated at 37°C for 72 h. The experiments were performed two times with seven mice per group, except the experiment in C (i–ii), which was performed three times with seven mice per group.
Similar articles
- TNF and lymphotoxin beta cooperate in the maintenance of secondary lymphoid tissue microarchitecture but not in the development of lymph nodes.
Kuprash DV, Alimzhanov MB, Tumanov AV, Anderson AO, Pfeffer K, Nedospasov SA. Kuprash DV, et al. J Immunol. 1999 Dec 15;163(12):6575-80. J Immunol. 1999. PMID: 10586051 - LTβR and CD40: working together in dendritic cells to optimize immune responses.
Gommerman JL, Summers deLuca L. Gommerman JL, et al. Immunol Rev. 2011 Nov;244(1):85-98. doi: 10.1111/j.1600-065X.2011.01056.x. Immunol Rev. 2011. PMID: 22017433 Review. - Expression of lymphotoxin beta governs immunity at two distinct levels.
Junt T, Tumanov AV, Harris N, Heikenwalder M, Zeller N, Kuprash DV, Aguzzi A, Ludewig B, Nedospasov SA, Zinkernagel RM. Junt T, et al. Eur J Immunol. 2006 Aug;36(8):2061-75. doi: 10.1002/eji.200626255. Eur J Immunol. 2006. PMID: 16841297 - The complementation of lymphotoxin deficiency with LIGHT, a newly discovered TNF family member, for the restoration of secondary lymphoid structure and function.
Wang J, Foster A, Chin R, Yu P, Sun Y, Wang Y, Pfeffer K, Fu YX. Wang J, et al. Eur J Immunol. 2002 Jul;32(7):1969-79. doi: 10.1002/1521-4141(200207)32:7<1969::AID-IMMU1969>3.0.CO;2-M. Eur J Immunol. 2002. PMID: 12115617 - Biology and signal transduction pathways of the Lymphotoxin-αβ/LTβR system.
Remouchamps C, Boutaffala L, Ganeff C, Dejardin E. Remouchamps C, et al. Cytokine Growth Factor Rev. 2011 Oct-Dec;22(5-6):301-10. doi: 10.1016/j.cytogfr.2011.11.007. Cytokine Growth Factor Rev. 2011. PMID: 22152226 Review.
Cited by
- Lymphotoxin-sensitive microenvironments in homeostasis and inflammation.
Boulianne B, Porfilio EA, Pikor N, Gommerman JL. Boulianne B, et al. Front Immunol. 2012 Jul 31;3:243. doi: 10.3389/fimmu.2012.00243. eCollection 2012. Front Immunol. 2012. PMID: 22866054 Free PMC article. - Treg engage lymphotoxin beta receptor for afferent lymphatic transendothelial migration.
Brinkman CC, Iwami D, Hritzo MK, Xiong Y, Ahmad S, Simon T, Hippen KL, Blazar BR, Bromberg JS. Brinkman CC, et al. Nat Commun. 2016 Jun 21;7:12021. doi: 10.1038/ncomms12021. Nat Commun. 2016. PMID: 27323847 Free PMC article. - The TNF receptor and Ig superfamily members form an integrated signaling circuit controlling dendritic cell homeostasis.
De Trez C, Ware CF. De Trez C, et al. Cytokine Growth Factor Rev. 2008 Jun-Aug;19(3-4):277-84. doi: 10.1016/j.cytogfr.2008.04.013. Epub 2008 Jun 3. Cytokine Growth Factor Rev. 2008. PMID: 18511331 Free PMC article. Review. - Ectopic lymphoid tissues and local immunity.
Carragher DM, Rangel-Moreno J, Randall TD. Carragher DM, et al. Semin Immunol. 2008 Feb;20(1):26-42. doi: 10.1016/j.smim.2007.12.004. Epub 2008 Feb 19. Semin Immunol. 2008. PMID: 18243731 Free PMC article. Review. - Development of secondary lymphoid organs.
Randall TD, Carragher DM, Rangel-Moreno J. Randall TD, et al. Annu Rev Immunol. 2008;26:627-50. doi: 10.1146/annurev.immunol.26.021607.090257. Annu Rev Immunol. 2008. PMID: 18370924 Free PMC article. Review.
References
- Mellman, I., and R.M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell. 106:255–258. - PubMed
- Quezada, S.A., L.Z. Jarvinen, E.F. Lind, and R.J. Noelle. 2004. CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 22:307–328. - PubMed
- Schulz, O., A.D. Edwards, M. Schito, J. Aliberti, S. Manickasingham, A. Sher, and C. Reis e Sousa. 2000. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 13:453–462. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials