Nucleation of protein fibrillation by nanoparticles - PubMed (original) (raw)
Nucleation of protein fibrillation by nanoparticles
Sara Linse et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Nanoparticles present enormous surface areas and are found to enhance the rate of protein fibrillation by decreasing the lag time for nucleation. Protein fibrillation is involved in many human diseases, including Alzheimer's, Creutzfeld-Jacob disease, and dialysis-related amyloidosis. Fibril formation occurs by nucleation-dependent kinetics, wherein formation of a critical nucleus is the key rate-determining step, after which fibrillation proceeds rapidly. We show that nanoparticles (copolymer particles, cerium oxide particles, quantum dots, and carbon nanotubes) enhance the probability of appearance of a critical nucleus for nucleation of protein fibrils from human beta(2)-microglobulin. The observed shorter lag (nucleation) phase depends on the amount and nature of particle surface. There is an exchange of protein between solution and nanoparticle surface, and beta(2)-microglobulin forms multiple layers on the particle surface, providing a locally increased protein concentration promoting oligomer formation. This and the shortened lag phase suggest a mechanism involving surface-assisted nucleation that may increase the risk for toxic cluster and amyloid formation. It also opens the door to new routes for the controlled self-assembly of proteins and peptides into novel nanomaterials.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
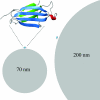
Fig. 1.
Size comparison of monomeric β2m (see Upper Left for enlargement) with nanoparticles. One protein molecule is placed on each particle in scale for size comparison with nanoparticles of 70 nm (Lower Left) and 200 nm (Right). This figure was prepared with Molmol (58).
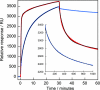
Fig. 2.
β2m fibrillation in the presence of nanoparticles. (A) Kinetics of β2m fibrillation. Thioflavin T fluorescence as a function of time for 80 μM (1 mg/ml) β2m at 37°C in 10 mM sodium phosphate buffer, pH 2.5, with 0.02% NaN3, without (black) or with 0.01 mg/ml nanoparticles with 85:15 (blue) or 50:50 (red) NIPAM/BAM ratio is shown. Smaller symbols are used for 70-nm particles. (B) Negative stain electron microscopy image of fibers grown in the presence of 70-nm 85:15 NIPAM/BAM copolymer nanoparticles. (Scale bar: 100 nm.)
Fig. 3.
Kinetics of β2m fibrillation. (A and E) Histograms of observed lag times. (B, D, and F) Thioflavin T fluorescence as a function of time showing kinetic traces for all samples in each group. (C) The average of all samples of each kind shown in D. (A and B) Forty micromolar β2m at 37°C in 20 mM sodium phosphate buffer, pH 2.5, with 50 mM NaCl and 0.02% NaN3. (C and D) Forty micromolar β2m at 37°C in 20 mM sodium phosphate buffer, pH 2.5, with 40 mM NaCl and 0.02% NaN3. (E and F) Forty micromolar β2m at 37°C in 20 mM sodium phosphate buffer, pH 2.5, with 50 mM NaCl and 0.02% NaN3. Color coding of kinetic traces and histograms: blue (0.01 mg/ml 70-nm 85:15 NIPAM/BAM particles), cyan (0.01 mg/ml 200-nm 85:15 NIPAM/BAM particles), red (0.01 mg/ml 70-nm 50:50 NIPAM/BAM particles), pink (0.01 mg/ml 200-nm 50:50 NIPAM/BAM particles), orange (100 nM 16-nm quantum dots), green (≤0.01 mg/ml 6-nm-diameter multiwalled carbon nanotubes), yellow (≤0.01 mg/ml 16-nm cerium oxide particles), and black (samples without particles).
Fig. 4.
SPR data for β2m associating to and dissociating from 200-nm 15:85 NIPAM/ BAM nanoparticles (blue) or 200-nm 50:50 NIPAM/BAM nanoparticles (red) linked to gold via a thiol group. The protein was injected between 0 and 30 min at constant concentration of 40 μM and followed by a constant buffer flow. The black curves were fitted to the association and dissociation data by using Eqs. 1 and 2 in
SI Text
. (Inset) An expansion of the dissociation data (blue) with fitted curve (black) for the 200-nm 15:85 NIPAM/BAM nanoparticles.
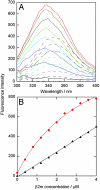
Fig. 5.
Change in β2m conformation in the presence of nanoparticles. (A) Trp fluorescence spectra of β2m titrated into buffer (dashed lines) and into a solution with 70-nm 50:50 NIPAM/BAM nanoparticles (solid lines). (B) Fluorescence intensity at 335 nm versus β2m concentration. β2m titrated into buffer (black triangles) fitted by a straight line, and β2m titrated into 70 nm 50:50 nanoparticles (red filled circles) fitted by a 1:1 binding curve is shown.
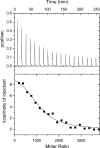
Fig. 6.
Isothermal titration calorimetry data at 5°C from titration of β2m into a solution containing 70-nm 50:50 NIPAM/BAM nanoparticles. The protein concentration was 160 μM, and the particle concentration was 1 mg/ml. Each injection was 15 μl with a total of 19 injections. (Upper) Raw data. (Lower) integrated data. The black line shows the fitted curve assuming a simple 1:1 binding model with one kind of sites (Eq. 3 in
SI Text
), with the parameter values Δ_H_ = 45 kJ/mol and KA = 4 × 105 M−1, n = 1,040.
Comment in
- Nanoparticles as catalysts for protein fibrillation.
Colvin VL, Kulinowski KM. Colvin VL, et al. Proc Natl Acad Sci U S A. 2007 May 22;104(21):8679-80. doi: 10.1073/pnas.0703194104. Epub 2007 May 14. Proc Natl Acad Sci U S A. 2007. PMID: 17502593 Free PMC article. No abstract available.
Similar articles
- Amyloid fibrillation kinetics: insight from atomistic nucleation theory.
Cabriolu R, Auer S. Cabriolu R, et al. J Mol Biol. 2011 Aug 5;411(1):275-85. doi: 10.1016/j.jmb.2011.05.032. Epub 2011 May 30. J Mol Biol. 2011. PMID: 21645521 - Heat of supersaturation-limited amyloid burst directly monitored by isothermal titration calorimetry.
Ikenoue T, Lee YH, Kardos J, Yagi H, Ikegami T, Naiki H, Goto Y. Ikenoue T, et al. Proc Natl Acad Sci U S A. 2014 May 6;111(18):6654-9. doi: 10.1073/pnas.1322602111. Epub 2014 Apr 21. Proc Natl Acad Sci U S A. 2014. PMID: 24753579 Free PMC article. - Mechanism of lysophosphatidic acid-induced amyloid fibril formation of beta(2)-microglobulin in vitro under physiological conditions.
Pál-Gábor H, Gombos L, Micsonai A, Kovács E, Petrik E, Kovács J, Gráf L, Fidy J, Naiki H, Goto Y, Liliom K, Kardos J. Pál-Gábor H, et al. Biochemistry. 2009 Jun 23;48(24):5689-99. doi: 10.1021/bi900356r. Biochemistry. 2009. PMID: 19432419 - Nanoparticles in relation to peptide and protein aggregation.
Zaman M, Ahmad E, Qadeer A, Rabbani G, Khan RH. Zaman M, et al. Int J Nanomedicine. 2014 Feb 12;9:899-912. doi: 10.2147/IJN.S54171. eCollection 2014. Int J Nanomedicine. 2014. PMID: 24611007 Free PMC article. Review. - Towards an understanding of the structural molecular mechanism of beta(2)-microglobulin amyloid formation in vitro.
Radford SE, Gosal WS, Platt GW. Radford SE, et al. Biochim Biophys Acta. 2005 Nov 10;1753(1):51-63. doi: 10.1016/j.bbapap.2005.07.006. Epub 2005 Aug 15. Biochim Biophys Acta. 2005. PMID: 16099226 Review.
Cited by
- The membrane axis of Alzheimer's nanomedicine.
Li Y, Tang H, Andrikopoulos N, Javed I, Cecchetto L, Nandakumar A, Kakinen A, Davis TP, Ding F, Ke PC. Li Y, et al. Adv Nanobiomed Res. 2021 Jan;1(1):2000040. doi: 10.1002/anbr.202000040. Epub 2020 Nov 26. Adv Nanobiomed Res. 2021. PMID: 33748816 Free PMC article. - Effects of Airborne Nanoparticles on the Nervous System: Amyloid Protein Aggregation, Neurodegeneration and Neurodegenerative Diseases.
von Mikecz A, Schikowski T. von Mikecz A, et al. Nanomaterials (Basel). 2020 Jul 10;10(7):1349. doi: 10.3390/nano10071349. Nanomaterials (Basel). 2020. PMID: 32664217 Free PMC article. Review. - Proteins Do Not Replicate, They Precipitate: Phase Transition and Loss of Function Toxicity in Amyloid Pathologies.
Ezzat K, Sturchio A, Espay AJ. Ezzat K, et al. Biology (Basel). 2022 Mar 30;11(4):535. doi: 10.3390/biology11040535. Biology (Basel). 2022. PMID: 35453734 Free PMC article. Review. - Targeting the mTOR Signaling Pathway Utilizing Nanoparticles: A Critical Overview.
Lunova M, Smolková B, Lynnyk A, Uzhytchak M, Jirsa M, Kubinová Š, Dejneka A, Lunov O. Lunova M, et al. Cancers (Basel). 2019 Jan 11;11(1):82. doi: 10.3390/cancers11010082. Cancers (Basel). 2019. PMID: 30642006 Free PMC article. Review. - Dimensionality of carbon nanomaterials determines the binding and dynamics of amyloidogenic peptides: multiscale theoretical simulations.
Todorova N, Makarucha AJ, Hine ND, Mostofi AA, Yarovsky I. Todorova N, et al. PLoS Comput Biol. 2013;9(12):e1003360. doi: 10.1371/journal.pcbi.1003360. Epub 2013 Dec 5. PLoS Comput Biol. 2013. PMID: 24339760 Free PMC article.
References
- European Commission. European Technology Platform on NanoMedicine, Vision Paper and Basis for a Strategic Research Agenda for NanoMedicine. Brussels: Eur Comm; 2005.
- Colvin VL. Nat Biotechnol. 2003;21:1166–1170. - PubMed
- Ramos-Nino ME, Scapoli L, Martinelli M, Land S, Mossman BT. Cancer Res. 2003;63:3539–3545. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials