CCR5 deficiency drives enhanced natural killer cell trafficking to and activation within the liver in murine T cell-mediated hepatitis - PubMed (original) (raw)
CCR5 deficiency drives enhanced natural killer cell trafficking to and activation within the liver in murine T cell-mediated hepatitis
Maureen N Ajuebor et al. Am J Pathol. 2007 Jun.
Abstract
Natural killer (NK) cells are innate immune cells that are enriched in the liver, but the processes underlying NK cell trafficking to the liver and cellular activation within the liver of patients with T cell-mediated liver diseases remain poorly defined. Concanavalin A (Con A) hepatitis is a murine model mimicking many aspects of human T cell-mediated liver diseases. Here we demonstrate that severe hepatitis in CCR5-deficient (KO) mice is associated with increased hepatic NK cell recruitment driven by enhanced hepatic production of CCL5 acting via CCR1 and by enhanced hepatic NK cell activation relative to that observed in wild-type mice after Con A administration. Furthermore, NK cell depletion ameliorated severe hepatitis in CCR5 KO mice but did not alter hepatitis in wild-type mice after Con A treatment. We propose that in the setting of CCR5 deficiency NK cells assume a profound effector role in Con A hepatitis via enhanced CCL5-CCR1 driven hepatic recruitment in addition to augmented cytokine-driven NK cell activation to produce interferon-gamma. These results highlight the potential profound impact of altered chemokine receptor expression on the innate immune response in the setting of T cell-mediated hepatitis.
Figures
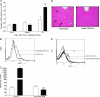
Figure 1
A: Time course of NK cell recruitment into the liver of WT (□; n = 5) and CCR5 KO (▪; n = 4) mice during Con A-induced hepatitis; **P < 0.01 versus all other groups. B: Representative FACS histogram depicting increased NK cell influx into the liver of CCR5 KO mice compared with WT mice at 8 hours after Con A administration. C: The effect of anti-asialo-GM1 Ab (n = 6) or control Ab (n = 5) treatment on ALT levels in WT and CCR5 KO mice 8 hours after Con A treatment; **P < 0.01 versus control Ab-treated WT mice; ##P < 0.01 versus anti-asialo Ab-treated CCR5 KO mice. D: Representative H&E staining of liver sections showing widespread/confluent hepatocellular necrosis (white arrows) and inflammatory cell infiltrates throughout the liver in Con A-treated CCR5 KO mice relative to patchy hepatocellular necrosis and mild inflammatory cell infiltrates in CCR5 KO mice pretreated with asialo-GM1 Ab all at 8 hours after Con A treatment. E: Representative FACS histogram depicting NK cell depletion after anti-asialo-GM1 mAb treatment in naïve CCR5 KO mice.
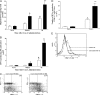
Figure 2
Correlation of severe hepatitis in CCR5 KO mice with enhanced hepatic IFN-γ production. A: Enzyme-linked immunosorbent assay determination of hepatic levels of IFN-γ in WT (□; n = 5) and CCR5 KO mice (▪; n = 4) during Con A-induced hepatitis. #P < 0.05 versus naïve WT; *P < 0.05 versus Con A-treated WT (90 minutes) and naïve groups; **P < 0.01 versus all WT groups. B: Number of IFN-γ-producing NK cells in the liver of WT (□; n = 6) and CCR5 KO (▪; n = 5) mice after Con A administration. *P < 0.05 versus Con-treated WT (90 minutes) and naïve groups; #P < 0.05 versus Con-treated WT (90 minutes) and naïve groups; **P < 0.01 versus all groups. C: Representative FACS dot plot demonstrating increased NK cell intracellular IFN-γ in the liver of CCR5 KO mice after 8 hours of Con A treatment relative to WT mice. D: Number of IFN-γ-producing CD4+ T cells in the liver of WT (□; n = 5) and CCR5 KO (▪; n = 4) mice 8 hours after Con A administration. *P < 0.05 versus naïve WT and naïve CCR5 KO mice; **P < 0.01 versus all other groups. E: Representative FACS histogram demonstrating reduced CD4+ T cell recruitment in the liver of CCR5 KO mice after anti-asialo-GM1 Ab treatment relative to CCR5 KO given control antibody.
Figure 3
A: Plasma ALT levels 8 hours after Con A administration in CCR5 KO mice pretreated with anti-IFN-γ mAb (▪; n = 4) relative to CCR5 KO mice given control Ab (▪; n = 4). ***P < 0.001 versus control Ab-treated CCR5 KO mice. B: Representative H&E staining of liver sections depicting widespread/confluent hepatocellular necrosis (arrows) and inflammatory cell infiltrates throughout the liver in Con A-treated CCR5 KO mice (arrows) relative to patchy hepatocellular necrosis and mild inflammatory cell infiltrates in CCR5 KO mice pretreated with anti-IFN-γ mAb all at 8 hours after Con A treatment. C: Effect of anti-IL-4 mAb treatment on hepatic IFN-γ levels in CCR5 KO 8 hours after Con A administration (▪; n = 4) relative to CCR5 KO mice given control Ab (▪; n = 4). *P < 0.05 versus control Ab-treated CCR5 KO mice. D: Number of NK cells in the liver 8 hours after Con A administration in CCR5 KO mice pretreated with anti-IL-4 mAb (▪; n = 4) relative to CCR5 KO mice given control Ab (▪; n = 4). *P < 0.05 versus control Ab-treated CCR5 KO mice. E: Representative FACS histogram depicting reduced NK cell recruitment in the liver of Con A-treated CCR5 KO mice at the 8 hour time point following pretreatment with anti-IL-4 mAb relative to Con A-treated CCR5 KO mice given control Ab. F: Number of IFN-γ-producing NK cells in the liver 8 hours after Con A administration in CCR5 KO mice pretreated with anti-IL-4 mAb (▪; n = 4) relative to CCR5 KO mice given control Ab (▪; n = 4). *P < 0.05 versus control Ab-treated CCR5 KO mice. G: Number of IFN-γ-producing NK cells in the liver 90 minutes after Con A administration in CCR5 KO mice pretreated with anti-IL-4 mAb (▪; n = 4) relative to CCR5 KO mice given control Ab (▪; n = 4). *P < 0.05 versus control Ab-treated CCR5 KO.
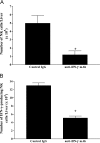
Figure 4
A: Number of NK cells in the liver 8 hours after Con A administration in CCR5 KO mice pretreated with anti-IFN-γ mAb (▪; n = 4) relative to CCR5 KO mice given control Ab (▪; n = 4). *P < 0.05 versus control Ab-treated CCR5 KO mice. B: Number of IFN-γ-producing NK cells in the liver 8 hours after Con A administration in CCR5 KO mice pretreated with anti-IFN-γ mAb (▪; n = 4) relative to CCR5 KO mice given control Ab (▪; n = 4). *P < 0.05 versus control Ab-treated CCR5 KO mice.
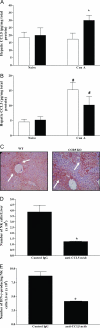
Figure 5
CCL5 promotes NK cell recruitment into the liver of CCR5 KO mice. A: Enzyme-linked immunosorbent assay determination of hepatic levels of CCL5 in WT (□; n = 5) and CCR5 KO mice (▪; n = 4) 8 hours after Con A-induced hepatitis. *P < 0.05 versus all other groups. B: Hepatic levels of CCL3 in WT (□; n = 5) and CCR5 KO mice (▪; n = 4) as determined by enzyme-linked immunosorbent assay at 8 hours after Con A-induced hepatitis. #P < 0.05 versus their respective naive groups. C: Immunohistochemical localization of CCL5 in paraffin-embedded liver sections from WT and CCR5 KO mice 8 hours after Con A administration (magnification, ×40). D: Number of NK cells in the liver 8 hours after Con A administration in CCR5 KO mice pretreated with anti-CCL5 mAb (▪; n = 4) relative to CCR5 KO mice given control Ab (▪; n = 4). *P < 0.05 versus control Ab-treated CCR5 KO mice. E: Number of IFN-γ-producing NK cells in the liver 8 hours after Con A administration in CCR5 KO mice pretreated with anti-CCL5 mAb (▪; n = 4) relative to CCR5 KO mice given control Ab (▪; n = 4). *P < 0.05 versus control Ab-treated CCR5 KO mice.
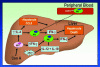
Figure 6
Schematic summary of events of how NK cells may promote severe hepatitis in CCR5-deficient mice following Con A administration. IL-4, in conjunction with IFN-γ, produced by activated hepatic NKT cells directly promotes early (ie, 90 minutes post-Con A) NK cell activation after Con A treatment (as reflected by increased NK cell IFN-γ production) and then indirectly mediate later (ie, 8 hours post-Con A) hepatic NK cell recruitment to and activation within the liver mediated by increased hepatic levels of hepatocyte-derived CCL5 acting via CCR1 expressed on NK cells. Activation of antigen-presenting cells during the development of Con A hepatitis may result in the increased production and release of IL-12 and IL-18 within the liver, and both of these cytokines may subsequently promote further NK cell activation and IFN-γ production within the liver. It is the combination of these events that drives the development of severe liver injury in CCR5-deficient mice following Con A administration.
Similar articles
- CCL3/MIP-1alpha is pro-inflammatory in murine T cell-mediated hepatitis by recruiting CCR1-expressing CD4(+) T cells to the liver.
Ajuebor MN, Hogaboam CM, Le T, Proudfoot AE, Swain MG. Ajuebor MN, et al. Eur J Immunol. 2004 Oct;34(10):2907-18. doi: 10.1002/eji.200425071. Eur J Immunol. 2004. PMID: 15368307 - Pre-activation of T lymphocytes by low dose of concanavalin A aggravates toll-like receptor-3 ligand-induced NK cell-mediated liver injury.
Wang J, Sun R, Wei H, Dong Z, Tian Z. Wang J, et al. Int Immunopharmacol. 2006 May;6(5):800-7. doi: 10.1016/j.intimp.2005.11.023. Epub 2006 Jan 9. Int Immunopharmacol. 2006. PMID: 16546711 - CCR5 deficiency exacerbates T-cell-mediated hepatitis in mice.
Moreno C, Gustot T, Nicaise C, Quertinmont E, Nagy N, Parmentier M, Le Moine O, Devière J, Louis H. Moreno C, et al. Hepatology. 2005 Oct;42(4):854-62. doi: 10.1002/hep.20865. Hepatology. 2005. PMID: 16175603 - CCR5 deficiency enhances hepatic innate immune cell recruitment and inflammation in a murine model of acute hepatitis B infection.
Stevens KE, Thio CL, Osburn WO. Stevens KE, et al. Immunol Cell Biol. 2019 Mar;97(3):317-325. doi: 10.1111/imcb.12221. Epub 2019 Jan 24. Immunol Cell Biol. 2019. PMID: 30536991 - Lack of chemokine receptor CCR5 promotes murine fulminant liver failure by preventing the apoptosis of activated CD1d-restricted NKT cells.
Ajuebor MN, Aspinall AI, Zhou F, Le T, Yang Y, Urbanski SJ, Sidobre S, Kronenberg M, Hogaboam CM, Swain MG. Ajuebor MN, et al. J Immunol. 2005 Jun 15;174(12):8027-37. doi: 10.4049/jimmunol.174.12.8027. J Immunol. 2005. PMID: 15944310
Cited by
- Dynamic regulation of innate lymphoid cells in the mucosal immune system.
Shao F, Yu D, Xia P, Wang S. Shao F, et al. Cell Mol Immunol. 2021 Jun;18(6):1387-1394. doi: 10.1038/s41423-021-00689-6. Epub 2021 May 12. Cell Mol Immunol. 2021. PMID: 33980994 Free PMC article. Review. - V(alpha)14iNKT cells promote liver pathology during adenovirus infection by inducing CCL5 production: implications for gene therapy.
Ajuebor MN, Chen Q, Strieter RM, Adegboyega PA, Aw TY. Ajuebor MN, et al. J Virol. 2010 Sep;84(17):8520-9. doi: 10.1128/JVI.00605-10. Epub 2010 Jun 23. J Virol. 2010. PMID: 20573836 Free PMC article. - Interference with oligomerization and glycosaminoglycan binding of the chemokine CCL5 improves experimental liver injury.
Nellen A, Heinrichs D, Berres ML, Sahin H, Schmitz P, Proudfoot AE, Trautwein C, Wasmuth HE. Nellen A, et al. PLoS One. 2012;7(5):e36614. doi: 10.1371/journal.pone.0036614. Epub 2012 May 4. PLoS One. 2012. PMID: 22574195 Free PMC article. - Efficacy of a Peruvian Botanical Remedy (Sabell A4+) for Treating Liver Disease and Protecting Gastric Mucosal Integrity.
Swain MG, Wallace JL, Tyrrell DL, Cabanillas J, Aung SKH, Liu H, Finnie-Carvalho L, Shrestha G, Semple HA, Green FHY. Swain MG, et al. Evid Based Complement Alternat Med. 2019 Oct 24;2019:5486728. doi: 10.1155/2019/5486728. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 31781272 Free PMC article. - The potential risks of C-C chemokine receptor 5-edited babies in bone development.
Xie Y, Zhan S, Ge W, Tang P. Xie Y, et al. Bone Res. 2019 Jan 29;7:4. doi: 10.1038/s41413-019-0044-0. eCollection 2019. Bone Res. 2019. PMID: 30701110 Free PMC article. Review. No abstract available.
References
- Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, Dayer JM. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–345. - PubMed
- Locati M, Murphy PM. Chemokines and chemokine receptors: biology and clinical relevance in inflammation and AIDS. Annu Rev Med. 1999;50:425–440. - PubMed
- Johnston B, Kim CH, Soler D, Emoto M, Butcher EC. Differential chemokine responses and homing patterns of murine TCR alpha beta NKT cell subsets. J Immunol. 2003;171:2960–2969. - PubMed
- Ajuebor MN, Aspinall AI, Zhou F, Le T, Yang Y, Urbanski SJ, Sidobre S, Kronenberg M, Hogaboam CM, Swain MG. Lack of chemokine receptor CCR5 promotes murine fulminant liver failure by preventing the apoptosis of activated CD1d-restricted NKT cells. J Immunol. 2005;174:8027–8037. - PubMed
- Dean M, Carrington M, Winkler C, Huttley GA, Smith MW, Allikmets R, Goedert JJ, Buchbinder SP, Vittinghoff E, Gomperts E, Donfield S, Vlahov D, Kaslow R, Saah A, Rinaldo C, Detels R, O’Brien SJ. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science. 1996;273:1856–1862. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials