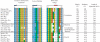Uracil-DNA glycosylases SMUG1 and UNG2 coordinate the initial steps of base excision repair by distinct mechanisms - PubMed (original) (raw)
Uracil-DNA glycosylases SMUG1 and UNG2 coordinate the initial steps of base excision repair by distinct mechanisms
Henrik Sahlin Pettersen et al. Nucleic Acids Res. 2007.
Abstract
DNA glycosylases UNG and SMUG1 excise uracil from DNA and belong to the same protein superfamily. Vertebrates contain both SMUG1 and UNG, but their distinct roles in base excision repair (BER) of deaminated cytosine (U:G) are still not fully defined. Here we have examined the ability of human SMUG1 and UNG2 (nuclear UNG) to initiate and coordinate repair of U:G mismatches. When expressed in Escherichia coli cells, human UNG2 initiates complete repair of deaminated cytosine, while SMUG1 inhibits cell proliferation. In vitro, we show that SMUG1 binds tightly to AP-sites and inhibits AP-site cleavage by AP-endonucleases. Furthermore, a specific motif important for the AP-site product binding has been identified. Mutations in this motif increase catalytic turnover due to reduced product binding. In contrast, the highly efficient UNG2 lacks product-binding capacity and stimulates AP-site cleavage by APE1, facilitating the two first steps in BER. In summary, this work reveals that SMUG1 and UNG2 coordinate the initial steps of BER by distinct mechanisms. UNG2 is apparently adapted to rapid and highly coordinated repair of uracil (U:G and U:A) in replicating DNA, while the less efficient SMUG1 may be more important in repair of deaminated cytosine (U:G) in non-replicating chromatin.
Figures
Figure 1.
Alignment of SMUG1 orthologs arranged in descending order of sequence similarity to hSMUG1. Sequences were obtained by TBLASTN 2.2.14 including GenBank, EMBL, DDBJ and PDB sequences (54). The alignment of the SMUG1 orthologs was generated using ClustalW (55); the final alignment was made with Jalview (56). Secondary structure of xSMUG1 is illustrated above the alignment. The alignment displays the important functional motifs characterizing the SMUG1 enzyme with its description above. Individual residues within each motif are coloured according to ClustalX colour coding. Species and accession number used are as follows: Hs (H. sapiens, AAL86910.1_), Pt (Pan troglodytes, XP_509109), Mm (M. musculus, Q6P5C5), Bt (Bos Taurus, Q59I47), Rn (Rattus norvegicus, Q811Q1), Cf (Canis familiaris, XP_543623.2), Tn (Tetraodon nigroviridis, CAF95523.1), Xl (Xenopus laevis, Q9YGN6), Gm (Geobacter metallireducens GS-15, YP_282069.1), AE (Azoarcus sp. EbN1, YP_158606.1),Ci (Ciona intestinalis, AK115076.1), Sp (Strongylocentrotus purpuratus, XP_782746.1), Rb (Rhodopirellula baltica SH 1, NP_869403.1), Tc (Tribolium castaneum, XP_971699.1), Ag (Anopheles gambiae str. PEST, XP_312038.2), Am (Apis mellifera, XP_396883.2), Dm (Drosophila melanogaster, NP_650609.1), Dp (Drosophila pseudoobscura, EAL272349.1)_.
Figure 2.
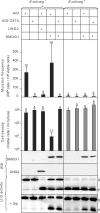
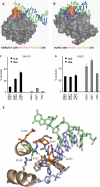
Mutation frequency and cell density in cultures of Ung- deficient and Ung-proficient E. coli ung- cells co-expressing AID and SMUG1 or UNG2. U:G mispairs were introduced into chromosomal DNA by expressing AID in E. coli ung- cells (black bars) and E. coli ung+ cells (grey bars). A catalytically inactive AID mutant (AID-C87A) and empty vector were included as controls. Mutation frequencies were measured by the rifampicin resistance assay (# rifR/108 cells). The error bars represent standard deviations calculated from the number of experiments given on top of each bar. Expression of SMUG1 and UNG2 was confirmed by western blot (WB) analysis and UDG activity were assayed on a U:G oligonucleotide substrate (U:G activity). Ugi was added (+Ugi) to the extracts to verify SMUG1 activity. Uncleaved substrate (S) and cleaved product (P) are indicated.
Figure 3
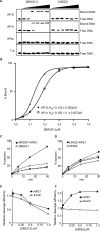
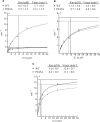
Product binding, effects of the AP-endonuclease APE1 and effects on AP-endonucleases APE1 and Exo III. (A) EMSA analysis of SMUG1 and UNG2 on AP-sites; 50, 100, 200, 300, 400 and 500 nM hSMUG1 and hUNG2 were incubated with 4 nM ds oligonucleotide (with complementary bases as indicated) or ss oligonucleotide, following uracil-excision binding to the product AP-sites were analysed by non-denaturating PAGE. (B) Percent bound AP:G and AP:A oligo plotted against the concentration of SMUG1 (µM) using a sigmoid curve fit model in GraphPad Prism®. The Kd values were calculated and represent the concentration of SMUG1 giving 50% of maximal binding to the AP-oligo. (C) Multiple turnover oligonucleotide assay with 0.85 nM SMUG1 and 20 nM U:G substrate (solid lines) or 20 nM Uss substrate (dotted lines) in the absence or presence of 8.5 nM APE1. (D) Multiple turnover oligonucleotide assay with 0.085 nM UNG2 and 20 nM U:G substrate (solid lines) or 20 nM Uss substrate (dotted lines) in the absence or presence of 8.5 nM APE1. Uracil excision was quantified after piperidine cleavage of the AP-site and separation by PAGE. (E) AP-cleavage activity of APE1 and ExoIII in the presence of SMUG1. (F) AP-cleavage activity of APE1 and ExoIII in the presence of UNG2. In (E) and (F), the AP-endonucleases (0.025 nM) were incubated for 10 min with 2 nM oligonucleotide substrate containing a central AP-site opposite guanine (AP:G) together with increasing amounts (0–1 µM) of SMUG1 or UNG2. AP-endonucleolytic cleavage was quantified after separation by PAGE.
Figure 4.
Alignment of important motifs and structural organization of active-site residues. (A) SMUG1 orthologs from human (AAL86910.1), mouse (XP_509109.1), δ proteo-bacteria (YP_383069.1) and sea urchin (XP_782746.1) aligned in ClustalW (55) with human UNG (NP_550433.1), human Cytomegalovirus (CMV) UNG (P16769), human TDG (NP_003202.3) and E. coli MUG (P0A9H1). Final alignment was made in Jalview (56) and individual residues coloured according to ClustalX colour codes. SMUG1 residues mutated in this work are indicated above. (B) Structural organization of the active sites of SMUG1 (PDB entry 1OE5) and human UNG (PDB entry 1SSP), showing key residues (numbered according the hSMUG1 orthologs) in catalysis, lesion binding and specificity.
Figure 5.
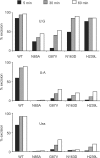
Uracil-substrate preference of SMUG1 active-site mutants. Limited turnover oligonucleotide assay of SMUG1 mutants. Equimolar amounts (20 nM) of enzyme and [33P]-labelled uracil oligonucleotide substrate (U:G, U:A and Uss) were incubated for 5, 30 and 60 min. Uracil excision was quantified after piperidine cleavage of the AP-site and separation by denaturing PAGE.
Figure 6.
DNA interactions and uracil-context dependence of SMUG1 and UNG. (A, B) Surface models of the DNA complex structure of xSMUG1 (PDB entry 1OE5) and hUNG (PDB entry 1EMH), respectively. The hSMUG1-specific motif 244-NPQANK-249 (xSMUG1 255-NPQANK-260) (orange) points towards the distal strand (green), while the conserved uracil-flipping motif—HPSPR/L—(pink) points towards the uracil-containing proximal strand (blue). (C) Sequence context dependence of hSMUG1. Multiple turnover oligonucleotide assays (0.4 nM SMUG1 and 20 nM substrate) on oligonucleotides with uracil in different sequence contexts. (D) Sequence context dependence of UNG2. Multiple turnover oligonucleotide assays (0.04 nM UNG2 and 20 nM substrate) on substrates with uracil in different sequence contexts. (E) Close-up presentation of the SMUG1 wedge motif (PDB entry 1OE5). Residues with side-chains facing the distal strand (hSMUG1-R243, -P245, -K249) are colored orange. hSMUG1-N248 (colored green) makes two hydrogen bonds (green dots) to hSMUG1-P242 and –R140, respectively. The residues are labeled according to the human SMUG1 sequence.
Figure 7.
Michaelis–Menten plots of SMUG1-WT and SMUG1-P245A. Each point is the mean value calculated from three independent experiments. Uracil excision at each point is below 30%. Kinetic parameters (Km, kcat) were calculated by the Wilkinson method using the Enzpack 1.4 (Biosoft) software package. (A) 1 nM SMUG1-WT and SMUG1-P245A measured on U:G oligonucleotide substrate (1–10 µM). (B) 20 nM SMUG1-WT and SMUG1-P245A measured on U:A oligonucleotide substrate (2–20 µM). (C) 5 nM SMUG1-WT and SMUG1-P245A measured on Uss oligonucleotide substrate (2–20 µM). Note the different values on the axis.
Figure 8.
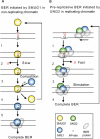
Repair of deaminated cytosine; model illustrating distinct coordination of BER initiated by SMUG1 and UNG2 in non-replicating chromatin and in replicating chromatin (foci), respectively. (A) 1. SMUG1 binds to the lesion and interacts with both strands in the DNA-helix. Uracil is probably flipped out of the helix and into the active site. 2. The catalysis is not very efficient because the active site is relaxed to be able to bind several other lesions. SMUG1 stays bound to the AP-site after excision. 3. APE1 competes with SMUG1 for AP-site binding. 4. SMUG1 is released form the product and is free to bind new lesions. 5. APE1 cuts the DNA strand, and Polβ/XRCC1/LigIIIα is recruited and completes BER. (B) 1. UNG2 is likely part of a highly coordinated and efficient repair complex scanning for lesions (U:G) in front of the replication fork (UNG2 is localized in replication foci). 2. Encountering the lesion uracil is flipped out of the DNA-helix and into the highly specific catalytic pocket of UNG2. Uracil is released by efficient hydrolysis of the _N_-glycosidic bond. Note, UNG2 interacts only with the uracil-containing DNA strand. UNG2 is immediately released from the AP-site and APE1 binds. 3. UNG2 stimulates APE1 cleavage of the AP-site and BER is completed.
Similar articles
- Strikingly different properties of uracil-DNA glycosylases UNG2 and SMUG1 may explain divergent roles in processing of genomic uracil.
Doseth B, Ekre C, Slupphaug G, Krokan HE, Kavli B. Doseth B, et al. DNA Repair (Amst). 2012 Jun 1;11(6):587-93. doi: 10.1016/j.dnarep.2012.03.003. Epub 2012 Apr 6. DNA Repair (Amst). 2012. PMID: 22483865 - Repair of U/G and U/A in DNA by UNG2-associated repair complexes takes place predominantly by short-patch repair both in proliferating and growth-arrested cells.
Akbari M, Otterlei M, Peña-Diaz J, Aas PA, Kavli B, Liabakk NB, Hagen L, Imai K, Durandy A, Slupphaug G, Krokan HE. Akbari M, et al. Nucleic Acids Res. 2004 Oct 12;32(18):5486-98. doi: 10.1093/nar/gkh872. Print 2004. Nucleic Acids Res. 2004. PMID: 15479784 Free PMC article. - Different organization of base excision repair of uracil in DNA in nuclei and mitochondria and selective upregulation of mitochondrial uracil-DNA glycosylase after oxidative stress.
Akbari M, Otterlei M, Peña-Diaz J, Krokan HE. Akbari M, et al. Neuroscience. 2007 Apr 14;145(4):1201-12. doi: 10.1016/j.neuroscience.2006.10.010. Epub 2006 Nov 13. Neuroscience. 2007. PMID: 17101234 - Uracil in DNA--occurrence, consequences and repair.
Krokan HE, Drabløs F, Slupphaug G. Krokan HE, et al. Oncogene. 2002 Dec 16;21(58):8935-48. doi: 10.1038/sj.onc.1205996. Oncogene. 2002. PMID: 12483510 Review. - Uracil in DNA--general mutagen, but normal intermediate in acquired immunity.
Kavli B, Otterlei M, Slupphaug G, Krokan HE. Kavli B, et al. DNA Repair (Amst). 2007 Apr 1;6(4):505-16. doi: 10.1016/j.dnarep.2006.10.014. Epub 2006 Nov 20. DNA Repair (Amst). 2007. PMID: 17116429 Review.
Cited by
- A unique uracil-DNA binding protein of the uracil DNA glycosylase superfamily.
Sang PB, Srinath T, Patil AG, Woo EJ, Varshney U. Sang PB, et al. Nucleic Acids Res. 2015 Sep 30;43(17):8452-63. doi: 10.1093/nar/gkv854. Epub 2015 Aug 24. Nucleic Acids Res. 2015. PMID: 26304551 Free PMC article. - Genetic variants in DNA repair pathway genes and risk of esophageal squamous cell carcinoma and gastric adenocarcinoma in a Chinese population.
Li WQ, Hu N, Hyland PL, Gao Y, Wang ZM, Yu K, Su H, Wang CY, Wang LM, Chanock SJ, Burdett L, Ding T, Qiao YL, Fan JH, Wang Y, Xu Y, Shi JX, Gu F, Wheeler W, Xiong XQ, Giffen C, Tucker MA, Dawsey SM, Freedman ND, Abnet CC, Goldstein AM, Taylor PR. Li WQ, et al. Carcinogenesis. 2013 Jul;34(7):1536-42. doi: 10.1093/carcin/bgt094. Epub 2013 Mar 15. Carcinogenesis. 2013. PMID: 23504502 Free PMC article. - The Multiple Cellular Roles of SMUG1 in Genome Maintenance and Cancer.
Raja S, Van Houten B. Raja S, et al. Int J Mol Sci. 2021 Feb 17;22(4):1981. doi: 10.3390/ijms22041981. Int J Mol Sci. 2021. PMID: 33671338 Free PMC article. Review. - The Role of Active-Site Residues Phe98, His239, and Arg243 in DNA Binding and in the Catalysis of Human Uracil-DNA Glycosylase SMUG1.
Iakovlev DA, Alekseeva IV, Vorobjev YN, Kuznetsov NA, Fedorova OS. Iakovlev DA, et al. Molecules. 2019 Aug 28;24(17):3133. doi: 10.3390/molecules24173133. Molecules. 2019. PMID: 31466351 Free PMC article. - Association of SMUG1 SNPs in Intron Region and Linkage Disequilibrium with Occurrence of Cervical Carcinoma and HPV Infection in Chinese Population.
Ye F, Wang H, Liu J, Cheng Q, Chen X, Chen H. Ye F, et al. J Cancer. 2019 Jan 1;10(1):238-248. doi: 10.7150/jca.27103. eCollection 2019. J Cancer. 2019. PMID: 30662544 Free PMC article.
References
- Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. - PubMed
- Barnes DE, Lindahl T. Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annu. Rev. Genet. 2004;38:445–476. - PubMed
- Neuberger MS, Harris RS, Di Noia J, Petersen-Mahrt SK. Immunity through DNA deamination. Trends Biochem. Sci. 2003;28:305–312. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous