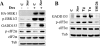The kinase inhibitor sorafenib induces cell death through a process involving induction of endoplasmic reticulum stress - PubMed (original) (raw)
The kinase inhibitor sorafenib induces cell death through a process involving induction of endoplasmic reticulum stress
Mohamed Rahmani et al. Mol Cell Biol. 2007 Aug.
Abstract
Sorafenib is a multikinase inhibitor that induces apoptosis in human leukemia and other malignant cells. Recently, we demonstrated that sorafenib diminishes Mcl-1 protein expression by inhibiting translation through a MEK1/2-ERK1/2 signaling-independent mechanism and that this phenomenon plays a key functional role in sorafenib-mediated lethality. Here, we report that inducible expression of constitutively active MEK1 fails to protect cells from sorafenib-mediated lethality, indicating that sorafenib-induced cell death is unrelated to MEK1/2-ERK1/2 pathway inactivation. Notably, treatment with sorafenib induced endoplasmic reticulum (ER) stress in human leukemia cells (U937) manifested by immediate cytosolic-calcium mobilization, GADD153 and GADD34 protein induction, PKR-like ER kinase (PERK) and eukaryotic initiation factor 2alpha (eIF2alpha) phosphorylation, XBP1 splicing, and a general reduction in protein synthesis as assessed by [35S]methionine incorporation. These events were accompanied by pronounced generation of reactive oxygen species through a mechanism dependent upon cytosolic-calcium mobilization and a significant decline in GRP78/Bip protein levels. Interestingly, enforced expression of IRE1alpha markedly reduced sorafenib-mediated apoptosis, whereas knockdown of IRE1alpha or XBP1, disruption of PERK activity, or inhibition of eIF2alpha phosphorylation enhanced sorafenib-mediated lethality. Finally, downregulation of caspase-2 or caspase-4 by small interfering RNA significantly diminished apoptosis induced by sorafenib. Together, these findings demonstrate that ER stress represents a central component of a MEK1/2-ERK1/2-independent cell death program triggered by sorafenib.
Figures
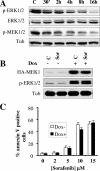
FIG. 1.
Inducible activation of the MEK/ERK pathway does not prevent sorafenib-mediated cell death. (A) U937 cells were exposed to 10 μM sorafenib for the designated intervals, after which cell lysates were obtained and subjected to Western blot analysis to monitor expression of ERK1/2, phospho-ERK1/2, and phospho-MEK1/2. For this and all subsequent Western blot analyses, blots were subsequently reprobed with antitubulin (Tub) antibodies to document equivalent loading and transfer. The results of a representative study are shown; two additional experiments yielded equivalent results. (B) Jurkat cells (MT6) inducibly expressing constitutively active HA-tagged MEK1 were left untreated or treated for 24 h with 2 μg/ml doxycycline (Dox). Cells were then exposed to 10 μM sorafenib (Sor) for an additional 4 h, after which protein lysates were prepared and analyzed for HA-MEK1 and phospho-ERK1/2 expression. Alternatively, cells were treated for 24 h, after which the extent of apoptosis was determined using an annexin V staining assay (C). Values represent the means ± standard deviations for at least three separate experiments performed in triplicate. C, control.
FIG. 2.
Exposure to sorafenib results in global translation inhibition. U937 cells were exposed to 10 μM sorafenib for the designated intervals and 0.5 μM thapsigargin or 0.5 μg/ml tunicamycin for 4 h and then pulsed with [35S]methionine for an additional 1 h. The cells were subsequently lysed, and protein lysates were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and transferred to a nitrocellulose membrane. The amounts of newly synthesized proteins were detected by autoradiography (A), and subsequently, the total amounts of proteins on the membranes were visualized by staining with ponceau S solution (B). The results shown are representative of three separate experiments.
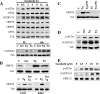
FIG. 3.
Sorafenib triggers the UPR in human leukemia cells. (A, upper) U937 cells were exposed to 10 μM sorafenib for the designated intervals, after which protein lysates were prepared and subjected to Western blot analysis using the indicated antibodies. (A, lower) U937 cells were exposed to 10 μM sorafenib (Sor), 0.5 μM thapsigargin (Tg), or 0.5 μg/ml tunicamycin (Tn) for 2 or 16 h, after which Western blot analysis was performed as described above. (B, upper) U937 cells were exposed to 10 μM sorafenib, 0.5 μM thapsigargin, or 0.5 μg/ml tunicamycin for 16 h, after which protein lysates were prepared and subjected to Western blot analysis to monitor GRP78 expression. (B, lower) U937 and K562 cells were exposed to sorafenib (10 μM) and thapsigargin (0.5 and 1 μM, respectively) alone or together for 6 h, after which protein lysates were prepared and subjected to Western blot analysis. (C) K562 cells were treated with 1 μM thapsigargin or 1 μg/ml tunicamycin in the presence or absence of 10 μM PD184352 for 16 h, after which protein lysates were prepared and subjected to Western blot analysis to monitor GRP78 expression. (D) K562 cells were exposed to 10 μM sorafenib for 4 h, after which cells were lysed and Western blot analysis was performed to monitor eIF2α phosphorylation and GADD153 expression. (E) Leukemia blasts were isolated from the peripheral blood of a patient with AML (FAB classification M2) and exposed to the designated concentration of sorafenib for 6 h, after which cells were lysed and protein was subjected to Western blot analysis to monitor eIF2α phosphorylation and GADD153 expression. For each experiment, at least two additional studies yielded equivalent results. C, control; Tub, antitubulin antibody.
FIG. 4.
Sorafenib triggers the UPR independently of MEK/ERK inactivation. (A) Jurkat cells (MT6) inducibly expressing constitutively active HA-tagged MEK1 were left untreated or treated for 24 h with 2 μg/ml doxycycline (Dox) and then exposed to 10 μM sorafenib (Sor) for an additional 4 h. Cells were then lysed, and Western blot analysis was performed using the designated antibodies. (B) U937 cells were treated with 10 μM sorafenib, 0.5 μM thapsigargin (Tg), 0.5 μg/ml tunicamycin (Tn), and 10 μM U0126 for 4 h, after which protein lysates were prepared and subjected to Western blot analysis using the indicated antibodies. For each experiment, at least two additional studies yielded equivalent results. C, control; Tub, antitubulin antibody.
FIG. 5.
Inhibition of PERK activity reduces eIF2α phosphorylation and enhances sorafenib-mediated cell death. (A, upper left) U937 cells were exposed to 10 μM sorafenib (Sor), 0.5 μM thapsigargin (Tg), or 0.5 μg/ml tunicamycin (Tn) for 16 h, after which protein lysates were prepared and subjected to Western blot analysis to monitor PERK phosphorylation. (A, upper right) K562 cells were transiently transfected with siRNA against PERK or with negative-control (NC) siRNA and incubated for 48 h, after which PERK mRNA levels were quantified by RT-PCR. Alternatively, transfected cells were treated with 10 μM sorafenib for 2 or 4 h, after which protein lysates were prepared and subjected to Western blot analysis to monitor eIF2α phosphorylation (A, lower). Alternatively, cells were treated for 24 h, after which the extent of cell death was monitored using annexin V staining (B). (C) Two clones (PERK-DN4 and PERK-DN7) of K562 cells stably expressing dominant-negative PERK and empty-vector cells (pBabe-puro) were treated with 1 μM thapsigargin for 2 h, after which protein lysates were prepared and subjected to Western blot analysis to monitor myc-tagged PERK and GADD153 protein expression. (D) Dominant-negative PERK clones and empty-vector K562 cells were treated with 10 μM sorafenib or 1 μM thapsigargin for 2 h, after which cell lysates were prepared and subjected to Western blot analysis. Alternatively, annexin V analysis was performed after 24 h of treatment to monitor the extent of cell death (E). For all annexin V studies, values represent the means ± standard deviations for at least three separate experiments performed in triplicate. *, significantly higher than values obtained for empty-vector pBabe-puro cells (P < 0.05). C, control; Tub, antitubulin antibody.
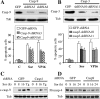
FIG. 6.
Sorafenib fully induces eIF2α phosphorylation in HRI, PKR, or GCN2 knockdown cells, an event that opposes sorafenib-mediated lethality. (A) K562 cells expressing V5-tagged eIF2α-DN (cl-9) or their controls (pcDNA3.1) were treated with sorafenib (Sor) or thapsigargin (Tg) for 2 h, after which protein lysates were prepared and subjected to Western blot analysis. Alternatively, the extent of apoptosis was determined using an annexin V staining assay after 24 h of exposure to sorafenib (B). Values represent the means for three separate experiments ± standard deviations. (C) Wild-type MEF (wt) in which eIF2α was intact and MEF cells in which endogenous eIF2α was genetically replaced by a nonphosphorylatable form of eIF2α (eIF2α ser 51/A) in both alleles were treated with 12 μM sorafenib for 24 h, after which the extent of cell death was determined using a 7-amino-actinomycin D assay (upper). Alternatively, cells were lysed at 3 and 6 h posttreatment and the protein lysates were subjected to Western blot analysis (lower). (D) Two clones (HRI-shRNA8 and HRI-shRNA18) of K562 cells stably transfected with an shRNA construct against HRI and cells transfected with an shRNA construct directed against eGFP were exposed to sorafenib for 2 h and 20 h, after which protein lysates were prepared and subjected to Western blot analysis to monitor HRI protein levels and eIF2α phosphorylation. (E) Two K562 clones (PKR2-RNAmir and PKR4-RNAmir) in which PKR was knocked down using shRNAmir and their control counterparts (eGFP-shRNAmir) were treated with sorafenib for 2 h and 20 h; then, PKR levels and eIF2α phosphorylation were monitored using Western blot analysis. An asterisk indicates nonspecific bands for HRI and PKR blots. (F) K562 cells were transiently transfected with siRNA against GCN2 or negative-control (NC) siRNA for 48 h. Cells were then exposed to 10 μM sorafenib for an additional 2 h, after which cells were lysed and protein lysates were subjected to Western blot analysis to monitor expression of GCN2 and eIF2α. C, control; Tub, antitubulin antibody.
FIG. 7.
Functional role of IRE1/XBP1 splicing and TRAF2/JNK1 in cell response to sorafenib. (A and B) U937 cells were exposed to sorafenib (Sor; 10 μM) for the designated intervals or thapsigargin (Tg; 0.5 μM) or tunicamycin (Tn; 0.5 μg/ml) for 16 h, after which cell lysates were obtained and subjected to Western blot analysis to monitor IRE1α (A) and spliced XBP1 (XBP1s; 50 kDa) (B, upper) protein levels. (B, lower) U937 and K562 cells were exposed to sorafenib (10 μM) for the designated intervals or tunicamycin (0.5 μg/ml for U937 and 1 μg/ml for K562 cells) for 16 h, after which XBP1 splicing (XBP1s, spliced; XBP1u, unspliced) was monitored using RT-PCR as described in Materials and Methods. (C) Two clones (shRNA7 and shRNA9) of U937 cells in which IRE1α was knocked down using shRNA and GFP-shRNA-transfected cells were treated with 7.5 μM sorafenib for 24, after which the extent of apoptosis was determined using an annexin V staining assay. (C, inset) Western blot analysis performed on lysates prepared from cells prior to treatment. (D, upper left) Western blot analysis performed on lysates prepared from two K562 cell clones (IRE1-cl7 and IRE1-cl13) expressing human HA-tagged IRE1α and their control counterparts (K562 cells transfected with pMSCVhyg empty vector). (D, upper right) XBP1 mRNA splicing was monitored by RT-PCR in IRE1-cl7 and their control empty-vector cells. (D, lower) IRE1-cl7, IRE1-cl13, and empty-vector cells were treated with 10 μM sorafenib for 28 h, after which the extent of apoptosis was determined using an annexin V staining assay. Values represent the means for three separate experiments ± standard deviations. *, significantly lower than values for empty-vector-transfected cells (P < 0.01). (E) U937 cells were transiently transfected with siRNA directed against XBP1 or negative-control (NC) siRNA for 24 h. Cells were then exposed to 7.5 μM sorafenib for an additional 24 h, after which cells were lysed and protein lysates were subjected to Western blot analysis to monitor expression of spliced XBP1. Alternatively, the extent of apoptosis was determined by monitoring annexin V staining. Values represent the means for three separate experiments ± standard deviations. *, significantly higher than values for NC transfected cells (P < 0.05). C, control; Tub, antitubulin antibody.
FIG. 8.
Treatment with sorafenib results in caspase-2 and caspase-4 processing. (A) U937 cells were exposed to 10 μM sorafenib (Sor) for the designated intervals or to 0.5 μM thapsigargin (Tg) or 0.5 μg/ml tunicamycin (Tn) for 16 h, after which protein lysates were prepared and subjected to Western blot analysis to monitor the protein levels of procaspase-2 (procasp-2) and procaspase-4 (procasp-2) and their cleavage products (c-casp-2 and c-casp-4, respectively). Note that high as well as low exposures of the blots were utilized to facilitate visualization of the decline in expression of the procaspases and the appearance of their cleavage fragments. (B) Leukemia blasts were isolated from the peripheral blood of a patient with AML (FAB classification M2) and exposed to the designated concentration of sorafenib (Sor) for 6 h, after which cells were lysed and protein was subjected to Western blot analysis to monitor caspase-2 processing. The blot was reprobed with ERK1/2 antibodies to document equivalent loading and transfer. (C) Jurkat cells were transiently transfected with siRNA directed against caspase-2 or negative-control (NC) siRNA for 24 h. Cells were then exposed to 10 μM sorafenib or 1 μM thapsigargin (Tg) for an additional 24 h, after which cells were lysed and protein lysates were subjected to Western blot analysis to monitor expression of caspase-2. Alternatively, the extent of apoptosis was determined using an annexin V staining assay. Values represent the means for three separate experiments ± standard deviations. *, significantly lower than values for NC transfected cells (P < 0.02). (D) Two U937 clones (casp4-shRNA3 and casp4-shRNA22) in which caspase-4 was knocked down and their control counterparts (GFP-shRNA) were monitored for expression of procaspase-4 by using Western blot analysis (upper). Alternatively, cells were exposed to 10 μM sorafenib (Sor) or 0.5 μM thapsigargin (Tg) for 24 h, after which the extent of cell death was monitored using an annexin V staining assay (lower). Values represent the means for three separate experiments ± standard deviations. * and **, significantly lower than values for GFP-shRNA cells (P < 0.02 and P < 0.01, respectively). C, control; Tub, antitubulin antibody.
FIG. 9.
Caspase-3 or caspase-9 knockdown modestly but significantly protects cells from sorafenib-mediated lethality. (A) Protein lysates were prepared from two clones of U937 cells transfected with caspase-9 shRNA (casp9-shRNA3 and casp9-shRNA8) and from GFP-shRNA-transfected cells and subjected to Western blot analysis (upper). Alternatively, these cells were treated with 10 μM sorafenib (Sor) or 2.5 μM VP16 for 24 h, after which the extent of apoptosis was determined using an annexin V staining assay (lower). (B) Protein lysates were prepared from two clones of U937 cells transfected with caspase-3 shRNA (casp3-shRNA10 and casp3-shRNA11) and from GFP-shRNA-transfected cells and subjected to Western blot analysis (upper). Alternatively, these cells were exposed to sorafenib (10 μM) and VP16 (2.5 μM) for 24 h and then subjected to an annexin V staining assay (lower). (C and D) Casp4-shRNA3 (C), casp9-shRNA8 (D), and GFP-shRNA cells were treated with sorafenib (10 μM) for the designated intervals or with thapsigargin (Tg; 0.5 μM) for 16 h, after which protein lysates were prepared and subjected to Western blot analysis. *, significantly less than values for controls (P < 0.05). C, control; Tub, antitubulin antibody.
FIG. 10.
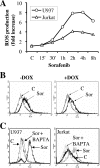
Treatment with sorafenib results in a potent calcium mobilization in leukemia cells. (A) U937 (left) and Jurkat (right) cells were loaded with Fluo3-AM for 30 min, after which cells were exposed to 10 μM sorafenib (Sor) for the designated intervals (U937) or 1 h (Jurkat), after which cytosolic calcium was monitored by flow cytometry as indicated in Materials and Methods. (B) U937 cells were loaded with Fluo3-AM for 30 min and then pretreated with thapsigargin (Tg) for 90 min, after which cells were exposed to 10 μM sorafenib for 1 h; then, the intensity of the fluorescence was monitored by flow cytometry. (C) Jurkat cells inducibly expressing constitutively active MEK1 were left untreated (left) or treated with 2 μg/ml doxycycline (DOX) (right) for 24 h and then loaded with Fluo3-AM for 30 min and exposed to 10 μM sorafenib (Sor) for an additional 1 h. The intensity of the fluorescence was then monitored by flow cytometry. For each experiment, the results of a representative study are shown; at least two additional experiments yielded equivalent results. C, control.
FIG. 11.
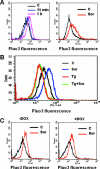
Exposure to sorafenib results in a dramatic increase in calcium-dependent ROS production. (A) U937 and Jurkat cells were exposed to 10 μM sorafenib (Sor) for the designated intervals, after which ROS production was monitored as indicated in Materials and Methods. Values represent the means for three separate experiments performed in triplicate and are expressed as increases (_n_-fold) relative to values for nontreated cells. (B) Jurkat cells inducibly expressing constitutively active MEK1 were left untreated (left) or treated with 2 μg/ml doxycycline (DOX) (right) for 24 h and then exposed to 10 μM sorafenib for an additional 1 h, after which ROS production was monitored as indicated in Materials and Methods. (C) U937 (left) and Jurkat (right) cells were treated with BAPTA-AM for 30 min before exposure to 10 μM sorafenib (Sor) for 1 h, after which ROS production was evaluated as described in Materials and Methods. C, control.
Similar articles
- Oxidative and endoplasmic reticulum stress signaling are involved in dehydrocostuslactone-mediated apoptosis in human non-small cell lung cancer cells.
Hung JY, Hsu YL, Ni WC, Tsai YM, Yang CJ, Kuo PL, Huang MS. Hung JY, et al. Lung Cancer. 2010 Jun;68(3):355-65. doi: 10.1016/j.lungcan.2009.07.017. Epub 2009 Aug 22. Lung Cancer. 2010. PMID: 19700217 - Apoptosis induced by the kinase inhibitor BAY 43-9006 in human leukemia cells involves down-regulation of Mcl-1 through inhibition of translation.
Rahmani M, Davis EM, Bauer C, Dent P, Grant S. Rahmani M, et al. J Biol Chem. 2005 Oct 21;280(42):35217-27. doi: 10.1074/jbc.M506551200. Epub 2005 Aug 18. J Biol Chem. 2005. PMID: 16109713 - ER stress, hypoxia tolerance and tumor progression.
Koumenis C. Koumenis C. Curr Mol Med. 2006 Feb;6(1):55-69. doi: 10.2174/156652406775574604. Curr Mol Med. 2006. PMID: 16472113 Review. - eIF2α phosphorylation as a biomarker of immunogenic cell death.
Kepp O, Semeraro M, Bravo-San Pedro JM, Bloy N, Buqué A, Huang X, Zhou H, Senovilla L, Kroemer G, Galluzzi L. Kepp O, et al. Semin Cancer Biol. 2015 Aug;33:86-92. doi: 10.1016/j.semcancer.2015.02.004. Epub 2015 Mar 6. Semin Cancer Biol. 2015. PMID: 25749194 Review.
Cited by
- Inositol-requiring enzyme 1alpha is a key regulator of angiogenesis and invasion in malignant glioma.
Auf G, Jabouille A, Guérit S, Pineau R, Delugin M, Bouchecareilh M, Magnin N, Favereaux A, Maitre M, Gaiser T, von Deimling A, Czabanka M, Vajkoczy P, Chevet E, Bikfalvi A, Moenner M. Auf G, et al. Proc Natl Acad Sci U S A. 2010 Aug 31;107(35):15553-8. doi: 10.1073/pnas.0914072107. Epub 2010 Aug 11. Proc Natl Acad Sci U S A. 2010. PMID: 20702765 Free PMC article. - Dysregulated autophagy contributes to caspase-dependent neuronal apoptosis.
Chung Y, Lee J, Jung S, Lee Y, Cho JW, Oh YJ. Chung Y, et al. Cell Death Dis. 2018 Dec 11;9(12):1189. doi: 10.1038/s41419-018-1229-y. Cell Death Dis. 2018. PMID: 30538224 Free PMC article. - Generalized convulsions due to sorafenib-induced hypocalcemia.
Cholongitas E, Georgousaki C, Spyrou S, Dasenaki M. Cholongitas E, et al. Indian J Gastroenterol. 2009 Jul-Aug;28(4):158-9. doi: 10.1007/s12664-009-0056-6. Indian J Gastroenterol. 2009. PMID: 19937413 No abstract available. - Combination strategies to overcome resistance to the BCL2 inhibitor venetoclax in hematologic malignancies.
Yue X, Chen Q, He J. Yue X, et al. Cancer Cell Int. 2020 Oct 29;20(1):524. doi: 10.1186/s12935-020-01614-z. Cancer Cell Int. 2020. PMID: 33292251 Free PMC article. Review.
References
- Ahmad, T., and T. Eisen. 2004. Kinase inhibition with BAY 43-9006 in renal cell carcinoma. Clin. Cancer Res. 2004. 10:6388S-6392S. - PubMed
- Boyce, M., and J. Yuan. 2006. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 13:363-373. - PubMed
- Brookes, P. S., Y. Yoon, J. L. Robotham, M. W. Anders, and S. S. Sheu. 2004. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 287:C817-C833. - PubMed
- Calfon, M., H. Zeng, F. Urano, J. H. Till, S. R. Hubbard, H. P. Harding, S. G. Clark, and D. Ron. 2002. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415:92-96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA100866/CA/NCI NIH HHS/United States
- R01 CA063753/CA/NCI NIH HHS/United States
- R01 CA093738/CA/NCI NIH HHS/United States
- CA 63753/CA/NCI NIH HHS/United States
- CA 100866/CA/NCI NIH HHS/United States
- CA 93738/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous