Regulation of ErbB-4 endocytosis by neuregulin in GABAergic hippocampal interneurons - PubMed (original) (raw)
Regulation of ErbB-4 endocytosis by neuregulin in GABAergic hippocampal interneurons
M Longart et al. Brain Res Bull. 2007.
Abstract
Neuregulin (NRG)/ErbB receptor signaling pathways have recently been implicated in the reversal of long-term potentiation at hippocampal glutamatergic synapses. Moreover, polymorphisms in NRG-1 and ErbB-4 genes have been linked to an increased risk for developing schizophrenia. ErbB-4 is highly expressed at glutamatergic synapses where it binds to PSD-95 via its carboxyl terminal T-V-V sequence. Here we investigated the expression, localization and trafficking of ErbB-4 in cultured hippocampal neurons by immunocytochemistry, surface protein biotinylation, and live labeling of native receptors. We show that neuronal ErbB-4 is detected at its highest levels in GABAergic interneurons, as observed in vivo. ErbB-4 immunoreactivity precedes PSD-95 expression, with ErbB-4 cluster initially forming in the absence of, but later associating with, PSD-95-positive puncta. By surface protein biotinylation, the fraction of ErbB-4 receptors on the plasma membrane increases from 30% to 65% between 6 and 16 days in vitro (DIV). Interestingly, 30 min of NRG stimulation triggers measurable ErbB-4 receptor internalization at DIV 16, despite increased colocalization with PSD-95. We also investigated the role of TNFalpha-converting enzyme (TACE)-mediated receptor processing in regulating ErbB-4 surface expression. We found that the cleavage-resistant JM-b isoform accounts for 80% of all ErbB-4 transcripts in cultured hippocampal neurons. Receptor stimulation or treatment with phorbol esters does not induce detectable ErbB-4 processing, indicating that neurons mostly rely on endocytosis of the intact receptor to regulate ErbB-4 surface expression. These results enhance our understanding of the regulation of ErbB-4--mediated signaling at glutamatergic synapses.
Figures
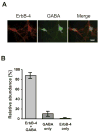
Figure 1. ErbB-4 is expressed in GABAergic interneurons
A, Double immunofluorescence labeling of DIV 14 hippocampal neurons with antibodies against ErbB-4 (red) and GABA (green). Scale bar = 20 μm. B, Quantitative analysis of co-expression of ErbB-4 and GABA. The sum of all cells positive for ErbB-4 or GABA was set as 100%. Data represent the mean ±SD of 1004 cells counted in 5 independent experiments.
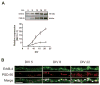
Figure 2. Regulation of ErbB-4 and PSD-95 expression in developing hippocampal neurons
A, (Top) Representative Western blots of whole cell lysates from DIV 5 – 21 neurons, probed with antibodies against ErbB-4 and PSD-95. Equal amounts of protein, as determined by a colorimetric protein assay (Bradford), were loaded in each lane. (Bottom) Quantitative analysis of Western blots. Relative expression levels of ErbB-4 (open circles) and PSD-95 (open rectangles) at DIV 5 were arbitrarily defined as 1. Each data point represents the mean ± SD of three independent experiments. B, Double immunofluorescence of ErbB-4 (green) and PSD-95 (red) in dendritic processes at DIV 5, 8, and 22. Arrows indicate areas of colocalization (yellow) between ErbB-4 and PSD-95. Scale bar = 5 μm.
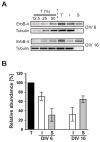
Figure 3. Surface expression of ErbB-4 in young and mature hippocampal neurons
A, Representative Western blots of surface-biotinylated DIV 6 and DIV 16 hippocampal neurons probed with a polyclonal antibody against the carboxyl terminus of ErbB-4. To control for linearity, 12.5%, 25%, and 50% fractions of the total protein input (T) before biotin / streptavidin pulldown were loaded in the first three lanes. I, supernatant after fractionation representing unbiotinylated internal proteins; S, pulldown fraction representing biotinylated surface proteins. To affirm that biotinylation was restricted to surface proteins, blots were re-probed with a monoclonal antibody against a cytosolic marker (tubulin). B, Quantitative analysis of ErbB-4 surface expression. ErbB-4 levels in total input fractions were set as 100%. Bars represent the mean ± S.D. from 7 and 6 independent xperiments for DIV 6 and DIV 16, respectively.
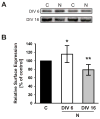
Figure 4. Surface expression of ErbB-4 in NRG-stimulated neurons
A, Representative Western blots of surface fractions from DIV 6 and DIV 16 neurons treated with 5 nM NRG1 peptide for 30 minutes and biotinylated as described in Figure 3. Two representative examples of surface ErbB-4 expression are shown for NRG-treated cultures (N) and vehicle-treated controls (C). B, Quantitative analysis of ErbB-4 surface expression in response to NRG peptide treatment. Surface ErbB-4 levels in controls were set as 100% (black bar), and compared to surface ErbB-4 levels after NRG treatment at DIV 6 (open bar) and DIV 16 (gray bar). Data represent the mean ± SD from 12 and 10 independent experiments for DIV 6 and DIV 16, respectively. *, p < 0.05; **, p < 0.01.
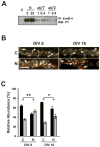
Figure 5. Internalization of activated ErbB-4 in live-labeled neurons
A, Effect of ab77 on ErbB-4 autophosphorylation in dissociated hippocampal neurons. DIV 8 neurons were incubated for 30 minutes at 37ºC with 1 or 0.4 μg/ml of mouse monoclonal antibodies ab77 or ab72, as indicated. ErbB-4 was immunoprecipitated using C-18 polyclonal antibody against the carboxyl terminus, and Western blots were probed for phosphotyrosine. For reference, neurons were stimulated with 5 or 25 nM NRG peptide as indicated. B. Double immunofluorescence of surface and internalized ErbB-4 receptors from representative dendritic areas of hippocampal neurons after NRG stimulation. Surface ErbB-4 receptors were labeled in live DIV 6 and DIV 16 neurons at 15ºC with Alexa 594-conjugated antibody ab77. Following NRG (N) or vehicle (C) treatment at 37ºC, cells were fixed and incubated with an Alexa 488-conjugated secondary antibody against mouse IgG. Yellow puncta (arrowheads) represent surface receptors and red puncta (arrows) represent receptors internalized during the treatment. Scale bar = 5 μm. C, Quantitative analysis of antibody feeding experiments. Data represent relative ErbB-4 abundance at the surface (black bars) and in internal compartments (gray bars). Values represent the mean ± SEM from 3 independent experiments (total number of ROIs analyzed: 99 (C) and 95 (N) for DIV 6; 94 (C) and 90 (N) for DIV 16). *, <0.05; **, <0.01.
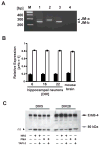
Figure 6. Cultured hippocampal neurons predominantly express the JM-b isoform and are resistant to NRG or phorbol ester-mediated processing
A, Representative RT-PCR of ErbB-4 JM-a/b splice variants. Lengths (in bp) of JM-a and JM-b PCR products are indicated (right). Lane 1, DIV 22 rat hippocampal neurons; lane 2, JM-a plasmid control; lane 3, JM-b plasmid control; lane 4, negative control; M, 100 bp DNA ladder. B, Expression of JM isoforms in hippocampal neurons cultured for different times (DIV 6, 16, 22), and in adult mouse whole brain. Relative expression levels were calculated from phosphorimager scans of RT-PCR products radioactively labeled with a probe common to both PCR products. Bars represent the mean ± SD of data collected from three different PCR cycles (cycles 26, 28, 30). C, Western blot analysis of ErbB-4 processing in NRG and PMA-stimulated neurons. DIV 5 and DIV 28 hippocampal neurons were treated for 30 minutes with 5 nM NRG peptide or 1μM PMA in the presence or absence of the TACE inhibitor TAPI-2 (12.5 μM). Proteins from cell lysates were blotted and probed with the C-18 antibody against the carboxyl terminus of ErbB-4. The black arrow indicates the molecular mass of the full-length ErbB-4 protein (180 kDa). The gray arrow marks the position of the expected 80 kDa fragment resulting from TACE-mediated proteolytic processing. The black arrowhead indicates a nonspecific band (ns) of approximately 70 kDa molecular mass that was present in all samples.
Similar articles
- Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons.
Ting AK, Chen Y, Wen L, Yin DM, Shen C, Tao Y, Liu X, Xiong WC, Mei L. Ting AK, et al. J Neurosci. 2011 Jan 5;31(1):15-25. doi: 10.1523/JNEUROSCI.2538-10.2011. J Neurosci. 2011. PMID: 21209185 Free PMC article. - Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses.
Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, Pelkey KA, Yang DJ, Xiong WC, Salter MW, Mei L. Huang YZ, et al. Neuron. 2000 May;26(2):443-55. doi: 10.1016/s0896-6273(00)81176-9. Neuron. 2000. PMID: 10839362 - The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses.
Garcia RA, Vasudevan K, Buonanno A. Garcia RA, et al. Proc Natl Acad Sci U S A. 2000 Mar 28;97(7):3596-601. doi: 10.1073/pnas.97.7.3596. Proc Natl Acad Sci U S A. 2000. PMID: 10725395 Free PMC article. - The neuregulin signaling pathway and schizophrenia: from genes to synapses and neural circuits.
Buonanno A. Buonanno A. Brain Res Bull. 2010 Sep 30;83(3-4):122-31. doi: 10.1016/j.brainresbull.2010.07.012. Epub 2010 Aug 3. Brain Res Bull. 2010. PMID: 20688137 Free PMC article. Review. - Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1.
Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Jaaro-Peled H, et al. Trends Neurosci. 2009 Sep;32(9):485-95. doi: 10.1016/j.tins.2009.05.007. Epub 2009 Aug 26. Trends Neurosci. 2009. PMID: 19712980 Free PMC article. Review.
Cited by
- ErbB4 in the brain: Focus on high grade glioma.
Pitcher JL, Alexander N, Miranda PJ, Johns TG. Pitcher JL, et al. Front Oncol. 2022 Aug 31;12:983514. doi: 10.3389/fonc.2022.983514. eCollection 2022. Front Oncol. 2022. PMID: 36119496 Free PMC article. Review. - Age-specific impacts of nicotine and withdrawal on hippocampal neuregulin signalling.
Keady J, Fisher M, Anderson E, LeMalefant R, Turner J. Keady J, et al. Eur J Neurosci. 2022 Sep;56(6):4705-4719. doi: 10.1111/ejn.15780. Epub 2022 Aug 30. Eur J Neurosci. 2022. PMID: 35899607 Free PMC article. - Functional Maturation of Human Stem Cell-Derived Neurons in Long-Term Cultures.
Lam RS, Töpfer FM, Wood PG, Busskamp V, Bamberg E. Lam RS, et al. PLoS One. 2017 Jan 4;12(1):e0169506. doi: 10.1371/journal.pone.0169506. eCollection 2017. PLoS One. 2017. PMID: 28052116 Free PMC article. - Molecular and cellular characterization of Neuregulin-1 type IV isoforms.
Shamir A, Buonanno A. Shamir A, et al. J Neurochem. 2010 Jun;113(5):1163-76. doi: 10.1111/j.1471-4159.2010.06677.x. Epub 2010 Mar 10. J Neurochem. 2010. PMID: 20218976 Free PMC article. - Dysregulated Signaling at Postsynaptic Density: A Systematic Review and Translational Appraisal for the Pathophysiology, Clinics, and Antipsychotics' Treatment of Schizophrenia.
de Bartolomeis A, Vellucci L, De Simone G, Mazza B, Barone A, Ciccarelli M. de Bartolomeis A, et al. Cells. 2023 Feb 10;12(4):574. doi: 10.3390/cells12040574. Cells. 2023. PMID: 36831241 Free PMC article.
References
- Adlkofer K, Lai C. Role of neuregulins in glial cell development. Glia. 2000;29:104–11. - PubMed
- Baulida J, Carpenter G. Heregulin degradation in the absence of rapid receptor-mediated internalization. Exp Cell Res. 1997;232:167–72. - PubMed
- Baulida J, Kraus MH, Alimandi M, Di FP, Carpenter G. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem. 1996;271:5251–7. - PubMed
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–79. - PubMed
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous