The Rad9-Hus1-Rad1 (9-1-1) clamp activates checkpoint signaling via TopBP1 - PubMed (original) (raw)
The Rad9-Hus1-Rad1 (9-1-1) clamp activates checkpoint signaling via TopBP1
Sinny Delacroix et al. Genes Dev. 2007.
Abstract
DNA replication stress triggers the activation of Checkpoint Kinase 1 (Chk1) in a pathway that requires the independent chromatin loading of the ATRIP-ATR (ATR-interacting protein/ATM [ataxia-telangiectasia mutated]-Rad3-related kinase) complex and the Rad9-Hus1-Rad1 (9-1-1) clamp. We show that Rad9's role in Chk1 activation is to bind TopBP1, which stimulates ATR-mediated Chk1 phosphorylation via TopBP1's activation domain (AD), a domain that binds and activates ATR. Notably, fusion of the AD to proliferating cell nuclear antigen (PCNA) or histone H2B bypasses the requirement for the 9-1-1 clamp, indicating that the 9-1-1 clamp's primary role in activating Chk1 is to localize the AD to a stalled replication fork.
Figures
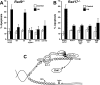
Figure 1.
Rad9 Ser387 is involved in Chk1 phosphorylation. (A) Constructs used in this study. Rad9 and TopBP1 are not drawn to scale. Rad9 PCNA-like N-terminal domain and tail are indicated. P in tail indicates intact phosphorylation sites, whereas A indicates phosphorylation sites mutated to Ala. BRCT domains in TopBP1 are indicated; AD is the activation domain. (B) Rad9−/− DT40 cells were transiently transfected with empty vector (EV) and vectors encoding untagged wild-type Rad9 (WT); Rad9-9A (9A), the mutant lacking nine C-terminal phosphorylation sites; and Rad9-9A to which the indicated phosphorylation sites were restored (denoted as Rad9-9A + site). Following treatment with 10 mM HU for 1 h, transfected _Rad9_−/− DT40 cells and parental (wild-type) DT40 cells were lysed, separated by SDS-PAGE, and sequentially immunoblotted for phospho-Ser345-Chk1, Chk1, and Rad9. The multiple bands present in the Rad9 immunoblots are due to various forms of phosphorylated Rad9 (Volkmer and Karnitz 1999). The dotted line indicates the juxtaposition of nonadjacent regions of the same gel. (C) Lysates from HEK293 cells transiently transfected with empty vector (EV) or vectors encoding S-tagged wild-type Rad9 (WT), Rad-9A (9A), or the indicated Rad9-9A add-back expression plasmids were precipitated with S-protein agarose beads. Bound proteins were sequentially immunoblotted for endogenous TopBP1 (top) and Rad9 (middle). The multiple bands present in the Rad9 immunoblots are due to various forms of phosphorylated Rad9 (Volkmer and Karnitz 1999). (Bottom) A portion of the lysate was also immunoblotted to demonstrate equal TopBP1 levels in all samples.
Figure 2.
TopBP1 BRCT domains 1 and 2 bind Rad9. (A) Schematic map of TopBP1 showing the BRCT domains, the AD, and the S-tagged constructs used in this study: full-length TopBP1 (FL); the N-terminal half of TopBP1 (N1/2); the C-terminal half of TopBP1 (C1/2); fragments encoding BRCT 1 and 2 (1 + 2), BRCT 3 (3), and BRCT 4 and 5 (4 + 5); and TopBP1 lacking BRCT 1 and 2 (Δ1 + 2). (B,D) HEK293 cells were transfected with empty vector (EV) or vectors encoding AU1-tagged Rad9 and the indicated S-tagged TopBP1 proteins shown in A. Lysates were precipitated with S-protein agarose beads. Bound proteins were sequentially immunoblotted for Rad9 (top) and S-tagged TopBP1 (middle). (Bottom) A portion of the lysate was immunoblotted with Rad9 to show equal expression. The multiple bands present in the Rad9 immunoblots are due to various forms of phosphorylated Rad9 (Volkmer and Karnitz 1999). (C) HEK293 cells were transfected with empty vector or a vector expressing S-tagged TopBP1 BRCT 1 and 2 domains (1 + 2). (Top) Lysates were precipitated with nonphosphorylated (Pep) or phosphorylated (P-Pep) Ser387 Rad9 peptide covalently linked to beads. (Bottom) A portion of the lysate was immunoblotted to demonstrate equal expression of the BRCT 1 + 2 domains.
Figure 3.
Rad9 activates Chk1 by binding TopBP1. _Rad9_−/− (A,B) or _Rad17_−/− (C_–_E) DT40 cells were transiently transfected with the indicated plasmids and treated with 10 mM HU for 1 h, and lysates were separated by SDS-PAGE and sequentially immunoblotted to detect phospho-Ser345-Chk1 and Chk1. To detect fusion protein and Rad17 expression, the samples were immunoblotted to detect S-tagged fusions (A_–_C,E), Rad17 (D), or PCNA (D). Transfections were with empty vector (EV); vectors expressing S-tagged wild-type Rad9 (WT), Rad9-9A (9A), Rad9-9A fused to full-length TopBP1 (9A–TopBP1), Rad9-9A–TopBP1 in which the WDDP motif in the AD was deleted from TopBP1 (9A–TopBP1–ΔWDDP), Rad9-9A fused to the TopBP1 AD (9A–AD), a tailless Rad9 fused to the AD (Δtail–AD), Rad17, or H2B fused to the TopBP1–AD (H2B–AD); or a vector expressing PCNA fused to the Rad9 tail (PCNA–Rad9 tail) or the TopBP1 AD (PCNA–AD). The multiple bands present in the Rad9 immunoblots are due to various forms of phosphorylated Rad9 (Volkmer and Karnitz 1999). Asterisk indicates nonspecific immunoreactive bands (A) or putative degradation products of the PCNA fusion proteins (D).
Figure 4.
Chk1 activation correlates with cell survival. Rad9−/− (A) or Rad17−/− (B) DT40 cells were cotransfected with the indicated plasmids (described in Fig. 3) and pEGFP-N1. The following day, EGFP-positive cells were purified by fluorescence-activated cell sorting, treated with HU, and stained with Hoechst 33258. For each sample, 250 cells were examined by microscopy. Apoptotic cells were identified based on nuclear morphology. Error bars indicate standard deviation of three to four independent experiments. (C) Model of Rad9’s role in Chk1 activation. See the text for details.
Similar articles
- Interaction between Rad9-Hus1-Rad1 and TopBP1 activates ATR-ATRIP and promotes TopBP1 recruitment to sites of UV-damage.
Ohashi E, Takeishi Y, Ueda S, Tsurimoto T. Ohashi E, et al. DNA Repair (Amst). 2014 Sep;21:1-11. doi: 10.1016/j.dnarep.2014.05.001. Epub 2014 May 27. DNA Repair (Amst). 2014. PMID: 25091155 - RHINO forms a stoichiometric complex with the 9-1-1 checkpoint clamp and mediates ATR-Chk1 signaling.
Lindsey-Boltz LA, Kemp MG, Capp C, Sancar A. Lindsey-Boltz LA, et al. Cell Cycle. 2015;14(1):99-108. doi: 10.4161/15384101.2014.967076. Cell Cycle. 2015. PMID: 25602520 Free PMC article. - Regulation of ATRIP protein abundance by RAD9 in the DNA damage repair pathway.
Peng XJ, Liu SJ, Bao CM, Liu YZ, Xie HW, Cai YH, Li BM, Hang HY, Ding X. Peng XJ, et al. Cell Mol Biol (Noisy-le-grand). 2015 Dec 9;61(8):31-6. Cell Mol Biol (Noisy-le-grand). 2015. PMID: 26667770 - Dial 9-1-1 for DNA damage: the Rad9-Hus1-Rad1 (9-1-1) clamp complex.
Parrilla-Castellar ER, Arlander SJ, Karnitz L. Parrilla-Castellar ER, et al. DNA Repair (Amst). 2004 Aug-Sep;3(8-9):1009-14. doi: 10.1016/j.dnarep.2004.03.032. DNA Repair (Amst). 2004. PMID: 15279787 Review.
Cited by
- DNA damage checkpoint execution and the rules of its disengagement.
Yam CQX, Lim HH, Surana U. Yam CQX, et al. Front Cell Dev Biol. 2022 Oct 6;10:1020643. doi: 10.3389/fcell.2022.1020643. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36274841 Free PMC article. Review. - ATR activation and replication fork restart are defective in FANCM-deficient cells.
Schwab RA, Blackford AN, Niedzwiedz W. Schwab RA, et al. EMBO J. 2010 Feb 17;29(4):806-18. doi: 10.1038/emboj.2009.385. Epub 2010 Jan 7. EMBO J. 2010. PMID: 20057355 Free PMC article. - MRN-dependent and independent pathways for recruitment of TOPBP1 to DNA double-strand breaks.
Montales K, Ruis K, Lindsay H, Michael WM. Montales K, et al. PLoS One. 2022 Aug 2;17(8):e0271905. doi: 10.1371/journal.pone.0271905. eCollection 2022. PLoS One. 2022. PMID: 35917319 Free PMC article. - Targeting telomeres: advances in telomere maintenance mechanism-specific cancer therapies.
Gao J, Pickett HA. Gao J, et al. Nat Rev Cancer. 2022 Sep;22(9):515-532. doi: 10.1038/s41568-022-00490-1. Epub 2022 Jul 5. Nat Rev Cancer. 2022. PMID: 35790854 Review. - Phospho-Ser/Thr-binding domains: navigating the cell cycle and DNA damage response.
Reinhardt HC, Yaffe MB. Reinhardt HC, et al. Nat Rev Mol Cell Biol. 2013 Sep;14(9):563-80. doi: 10.1038/nrm3640. Nat Rev Mol Cell Biol. 2013. PMID: 23969844 Review.
References
- Abraham R.T. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes & Dev. 2001;15:2177–2196. - PubMed
- Ball H.L., Ehrhardt M.R., Mordes D.A., Glick G.G., Chazin W.J., Cortez D., Ehrhardt M.R., Mordes D.A., Glick G.G., Chazin W.J., Cortez D., Mordes D.A., Glick G.G., Chazin W.J., Cortez D., Glick G.G., Chazin W.J., Cortez D., Chazin W.J., Cortez D., Cortez D. Function of a conserved checkpoint recruitment domain in ATRIP proteins. Mol. Cell. Biol. 2007;27:3367–3377. - PMC - PubMed
- Bermudez V.P., Lindsey-Boltz L.A., Cesare A.J., Maniwa Y., Griffith J.D., Hurwitz J., Sancar A., Lindsey-Boltz L.A., Cesare A.J., Maniwa Y., Griffith J.D., Hurwitz J., Sancar A., Cesare A.J., Maniwa Y., Griffith J.D., Hurwitz J., Sancar A., Maniwa Y., Griffith J.D., Hurwitz J., Sancar A., Griffith J.D., Hurwitz J., Sancar A., Hurwitz J., Sancar A., Sancar A. Loading of the human 9–1–1 checkpoint complex onto DNA by the checkpoint clamp loader hRad17–replication factor C complex in vitro. Proc. Natl. Acad. Sci. 2003;100:1633–1638. - PMC - PubMed
- Byun T.S., Pacek M., Yee M.C., Walter J.C., Cimprich K.A., Pacek M., Yee M.C., Walter J.C., Cimprich K.A., Yee M.C., Walter J.C., Cimprich K.A., Walter J.C., Cimprich K.A., Cimprich K.A. Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint. Genes & Dev. 2005;19:1040–1052. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous