Metal-free superoxide dismutase forms soluble oligomers under physiological conditions: a possible general mechanism for familial ALS - PubMed (original) (raw)
Metal-free superoxide dismutase forms soluble oligomers under physiological conditions: a possible general mechanism for familial ALS
Lucia Banci et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder selectively affecting motor neurons; 90% of the total cases are sporadic, but 2% are associated with mutations in the gene coding for the antioxidant enzyme copper-zinc superoxide dismutase (SOD1). The causes of motor neuron death in ALS are poorly understood in general, but for SOD1-linked familial ALS, aberrant oligomerization of SOD1 mutant proteins has been strongly implicated. In this work, we show that wild-type human SOD1, when lacking both its metal ions, forms large, stable, soluble protein oligomers with an average molecular mass of approximately 650 kDa under physiological conditions, i.e., 37 degrees C, pH 7.0, and 100 microM protein concentration. It further is shown here that intermolecular disulfide bonds are formed during oligomerization and that Cys-6 and Cys-111 are implicated in this bonding. The formation of the soluble oligomers was monitored by their ability to enhance the fluorescence of thioflavin T, a benzothiazole dye that increases in fluorescence intensity upon binding to amyloid fibers, and by disruption of this binding upon addition of the chaotropic agent guanidine hydrochloride. Our results suggest a general, unifying picture of SOD1 aggregation that could operate when wild-type or mutant SOD1 proteins lack their metal ions. Although we cannot exclude other mechanisms in SOD1-linked familial ALS, the one proposed here has the strength of explaining how a large and diverse set of SOD1 mutant proteins all could lead to disease through the same mechanism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
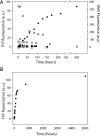
Fig. 1.
Formation of ThT-binding structures occurs concomitantly with disappearance of free cysteines when apo SOD1 is incubated at 37°C. (A) Left axis: Fluorescence caused by ThT binding to SOD1 [presented as arbitrary units (a.u.)] for fully metallated SOD1 (●), apo SOD1 (▲), copper-free SOD1 (○), apo AS SOD1 (□), and fully metallated AS SOD1 (■) during the incubation of the samples at 37°C. Right axis: Change in fluorescence of AMS bound to the free cysteines of the apo SOD1 WT sample (△) during its incubation at 37°C. (B) ThT-binding fluorescence of the apo SOD1 protein over 7 months of incubation.
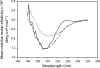
Fig. 2.
CD analysis. CD spectra of the fully metallated WT SOD1 protein (——), the apo WT SOD1 (– – –), and the aggregated species eluted from the chromatographic column of the incubated apo WT SOD1 (· · · · · ·). The higher percentage of β secondary structure in the apo WT SOD1 sample after incubation is evidenced by the increased contribution to the overall CD spectrum of the negative band at 218 nm, which is characteristic of β-sheet structure.
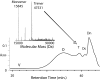
Fig. 3.
Size-exclusion chromatography and mass spectrometric detection of covalent oligomers of WT SOD1 during aggregation. Nondenaturing size-exclusion chromatography was used to analyze a sample of SOD1 apo protein after a 5-day incubation at 37°C. The sample exhibits a broad peak centered ≈34 min, corresponding to a molecular mass of ≈400 kDa (labeled O, oligomer), with geometry suggestive of a heterogeneous range of species, some as large as 1 MDa. The void volume is labeled V. Control SOD1 is dimeric (42 min, chromatogram not shown), and a population is seen in the 5-day sample (labeled Dn, noncovalent dimer). The 5-day sample also shows evidence of intermediate absorbance peaks at 39 min (labeled T, trimer) and 40 min (labeled Dc, covalent dimer) that might be intermediates in the oligomerization process. Fractions (O, T, Dc, and Dn) subsequently were analyzed by ESI-MS under denaturing conditions before or after reduction (
SI Fig. 6
and Inset). (Inset) The ESI mass spectrum recorded under nonreducing denaturing conditions showing a covalent trimeric form of SOD1 from the 39-min fraction above. Shown is the covalent profile of the molecule after zero-charge deconvolution with monomeric and trimeric forms of the protein. Reduction of all four fractions (O, T, Dc, and Dn) results in detection of monomeric species only (see
SI Fig. 6
for the complete experiment).
Fig. 4.
Proposed mechanism for SOD1 aggregation. Possible mechanism for in vivo formation of soluble oligomers that occurs when apo WT SOD1 protein is kept close to physiological conditions (37°C, 100 μM, and pH 7) for an extended period. The dark gray shapes represent free cysteines (Cys-6 and Cys-111), and the black triplets of parallel bars represent amyloid-like arrangements of hydrogen bonds. In the absence of metal ions, SOD1 proteins form abnormal disulfide cross-links and noncovalent associations with other SOD1 monomers or dimers.
Similar articles
- The Disulfide Bond, but Not Zinc or Dimerization, Controls Initiation and Seeded Growth in Amyotrophic Lateral Sclerosis-linked Cu,Zn Superoxide Dismutase (SOD1) Fibrillation.
Chattopadhyay M, Nwadibia E, Strong CD, Gralla EB, Valentine JS, Whitelegge JP. Chattopadhyay M, et al. J Biol Chem. 2015 Dec 18;290(51):30624-36. doi: 10.1074/jbc.M115.666503. Epub 2015 Oct 28. J Biol Chem. 2015. PMID: 26511321 Free PMC article. - Disruption of mitochondrial membrane integrity induced by amyloid aggregates arising from variants of SOD1.
Oladzad Abbasabadi A, Javanian A, Nikkhah M, Meratan AA, Ghiasi P, Nemat-Gorgani M. Oladzad Abbasabadi A, et al. Int J Biol Macromol. 2013 Oct;61:212-7. doi: 10.1016/j.ijbiomac.2013.07.007. Epub 2013 Jul 17. Int J Biol Macromol. 2013. PMID: 23872456 - Mitochondrial membrane disruption by aggregation products of ALS-causing superoxide dismutase-1 mutants.
Salehi M, Nikkhah M, Ghasemi A, Arab SS. Salehi M, et al. Int J Biol Macromol. 2015 Apr;75:290-7. doi: 10.1016/j.ijbiomac.2015.01.022. Epub 2015 Jan 16. Int J Biol Macromol. 2015. PMID: 25600987 - Mutant SOD1 instability: implications for toxicity in amyotrophic lateral sclerosis.
Tiwari A, Hayward LJ. Tiwari A, et al. Neurodegener Dis. 2005;2(3-4):115-27. doi: 10.1159/000089616. Neurodegener Dis. 2005. PMID: 16909016 Review. - Aggregation of copper-zinc superoxide dismutase in familial and sporadic ALS.
Chattopadhyay M, Valentine JS. Chattopadhyay M, et al. Antioxid Redox Signal. 2009 Jul;11(7):1603-14. doi: 10.1089/ars.2009.2536. Antioxid Redox Signal. 2009. PMID: 19271992 Free PMC article. Review.
Cited by
- Human superoxide dismutase 1 (hSOD1) maturation through interaction with human copper chaperone for SOD1 (hCCS).
Banci L, Bertini I, Cantini F, Kozyreva T, Massagni C, Palumaa P, Rubino JT, Zovo K. Banci L, et al. Proc Natl Acad Sci U S A. 2012 Aug 21;109(34):13555-60. doi: 10.1073/pnas.1207493109. Epub 2012 Aug 6. Proc Natl Acad Sci U S A. 2012. PMID: 22869735 Free PMC article. - Structural changes to monomeric CuZn superoxide dismutase caused by the familial amyotrophic lateral sclerosis-associated mutation A4V.
Schmidlin T, Kennedy BK, Daggett V. Schmidlin T, et al. Biophys J. 2009 Sep 16;97(6):1709-18. doi: 10.1016/j.bpj.2009.06.043. Biophys J. 2009. PMID: 19751676 Free PMC article. - Cholesterol secosterol aldehyde adduction and aggregation of Cu,Zn-superoxide dismutase: Potential implications in ALS.
Dantas LS, Chaves-Filho AB, Coelho FR, Genaro-Mattos TC, Tallman KA, Porter NA, Augusto O, Miyamoto S. Dantas LS, et al. Redox Biol. 2018 Oct;19:105-115. doi: 10.1016/j.redox.2018.08.007. Epub 2018 Aug 16. Redox Biol. 2018. PMID: 30142602 Free PMC article. - Cadmium effects on superoxide dismutase 1 in human cells revealed by NMR.
Polykretis P, Cencetti F, Donati C, Luchinat E, Banci L. Polykretis P, et al. Redox Biol. 2019 Feb;21:101102. doi: 10.1016/j.redox.2019.101102. Epub 2019 Jan 8. Redox Biol. 2019. PMID: 30654299 Free PMC article. - Novel chemical inhibitor against SOD1 misfolding and aggregation protects neuron-loss and ameliorates disease symptoms in ALS mouse model.
Woo TG, Yoon MH, Kang SM, Park S, Cho JH, Hwang YJ, Ahn J, Jang H, Shin YJ, Jung EM, Ha NC, Kim BH, Kwon Y, Park BJ. Woo TG, et al. Commun Biol. 2021 Dec 15;4(1):1397. doi: 10.1038/s42003-021-02862-z. Commun Biol. 2021. PMID: 34912047 Free PMC article.
References
- Ross CA, Poirier MA. Nat Rev Mol Cell Biol. 2005;6:891–898. - PubMed
- Stefani M, Dobson CM. J Mol Med. 2003;81:678–699. - PubMed
- Molina-Holgado F, Hider RC, Gaeta A, Williams R, Francis P. Biometals. 2007;20:639–654. - PubMed
- Bruijn LI, Miller TM, Cleveland DW. Annu Rev Neurosci. 2004;27:723–749. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous