DeltaNp63 regulates thymic development through enhanced expression of FgfR2 and Jag2 - PubMed (original) (raw)
DeltaNp63 regulates thymic development through enhanced expression of FgfR2 and Jag2
Eleonora Candi et al. Proc Natl Acad Sci U S A. 2007.
Abstract
p63, a homologue of the tumor suppressor p53, is pivotal for epithelial development, because its loss causes severe epithelial dysgenesis, although no information is so far available on the role of p63 in the thymus. We identified the expression of all p63 isoforms in the developing thymus. The p63(-/-) thymi show severe abnormalities in size and cellularity, even though the organ expresses normal levels of keratins 5 and 8, indicating a p63-independent differentiation of thymic epithelial cells (TEC). TEC were sufficiently developed to allow a significant degree of education to produce CD4/CD8 single- and double-positive T cells. To study the selective contribution of transactivation-active p63 (TAp63) and amino-deleted p63 (DeltaNp63) isoforms to the function of the TEC, we genetically complemented p63(-/-) mice by crossing p63(+/-) mice with transgenic mice expressing either TAp63alpha or DeltaNp63alpha under the control of the keratin 5 promoter. Thymic morphology and cellularity were partially restored by complementation with DeltaNp63, but not TAp63, one downstream effector being fibroblast growth factor receptor 2-IIIb (FgfR2-IIIb). Indeed, FgfR2-IIIb is regulated directly by p63, via its interaction with apobec-1-binding protein-1, and its knockout shows thymic defects similar to those observed in p63(-/-) thymi. In addition, expression of Jag2, a component of the Notch signaling pathway known to be required for thymic development, was enhanced by p63 in vivo genetic complementation. Like Jag2(-/-) thymi, p63(-/-) thymi also show reduced gammadelta cell formation. Therefore, p63, and particularly the DeltaNp63 isoform, is essential for thymic development via enhanced expression of FgfR2 and Jag2. The action of DeltaNp63 is not due to a direct regulation of TEC differentiation, but it is compatible with maintenance of their "stemness," the thymic abnormalities resulting from epithelial failure due to loss of stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
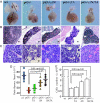
Fig. 1.
Fetal thymic development in p63−/− and genetically complemented mice. (A) Relative size of thymi (E19.5 embryos), dissected with the heart attached to provide a frame of reference. Thymi from p63−/− animals are reduced in size and exhibit severe structural anomalies. (B) Thymic histology (E19.5 sections stained with hematoxylin–eosin) in p63−/− mice before and after the reintroduction of TAp63α and/or ΔNp63α. (Scale bars, 300 μm.) (C) Histology of thymic cortical area in p63−/− mice before and after the reintroduction of TAp63α and/or ΔNp63α at bigger magnification. (Scale bars, 10 μm.) (D) The thymus/heart (E19.5 embryos) ratio of p63−/−, and p63−/−;TA mice was markedly reduced compared with wild type (wt), and increased in p63−/−;ΔN and p63−/−;ΔN;TA mice (n = 11; P value is indicated). (E) Thymic cell number (mechanical extraction from thymus from E19.5 embryos) in the different genotypes. Bar indicates standard deviation (n = 11; statistical significance is indicated).
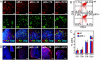
Fig. 2.
Histochemistry of keratins, cell death detection, and T cell counts in complemented mice. (A) Confocal staining of fetal thymus at E19.5 in wild-type (wt); p63−/− mice; and complemented p63−/−;ΔN, p63−/−;TA, and p63−/−;ΔN;TA mice. (Top and Middle) Tissue was stained for K5 (red) (Top) or K8 (green) (Middle). (Bottom) Merged fluorescence with DAPI (blue). The expression of K5 and K8 has a similar pattern in wild-type, p63−/−, and genetically complemented mice. (Scale bars, 60 μm.) (B and C) Staining for CD8 and CD4 in cells extracted from the thymi obtained from seven different mice for each genotype, wild type or p63−/−. Differences between control (wt) and p63−/− (knockout) genotypes for each particular subset were not statistically significant; bar indicates standard deviation. (D) Cell death was evaluated by TUNEL positivity (red); samples are as in A. In the thymi of p63−/− mice, a higher incidence of cell death is detected. The reintroduction of TAp63α results in an increase of cell death. The reintroduction of ΔNp63α results in a slight improvement of both parameters. The experiment was performed three times, and a representative experiment is shown. (Scale bars, 60 μm.)
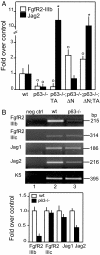
Fig. 3.
Expression of FgfR2 and Jag2 in complemented mice. (A) Quantitative real-time PCR indicates that the p63−/− reduced FgfR2-IIIb expression was restored upon reintroduction of ΔNp63α, but not TAp63α, whereas Jag2 expression was restored upon reintroduction of TAp63α and ΔNp63α [∗, 0.001 < P < 0.01 between wild-type (wt) and genetically complemented mice; ○, 0.01 < P < 0.05 between wt and genetically complemented mice; n = 7]. (B) (Upper) Semiquantitative RT-PCR for FgfR2 splicing variants IIIc and IIIb and for Jag1 and Jag2. The results indicate that only the expression of the IIIb isoform is down-regulated in the p63−/− thymus. Among the Notch ligands, the expression of Jag2 is more markedly reduced in comparison with Jag1. (Lower) Quantification of the semiquantitative RT-PCR (n = 5).
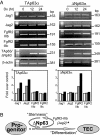
Fig. 4.
Expression of FgfR2, Jag1, and Jag2 in TAp63 and ΔNp63 Saos-2 Tet-on inducible cell lines. (A) Semiquantitative RT-PCR in Saos-2 Tet-on inducible cell lines (Dx, 2 μg/ml) overexpressing TAp63α or ΔNp63α isoforms. Actin is shown in the bottom image as a control. FgfR2, Jag1, and Jag2 expression were normalized to actin by densitometry, and results are reported as fold change over zero time (see graphics below actins). We performed three independent experiments, and a representative one is shown. Results obtained from the densitometry are shown as mean from three independent experiments. (B) Scheme of the role of ΔNp63 in “stemness” of thymic development. ∗, Jag2 is also induced by TAp63.
Similar articles
- Differential roles of p63 isoforms in epidermal development: selective genetic complementation in p63 null mice.
Candi E, Rufini A, Terrinoni A, Dinsdale D, Ranalli M, Paradisi A, De Laurenzi V, Spagnoli LG, Catani MV, Ramadan S, Knight RA, Melino G. Candi E, et al. Cell Death Differ. 2006 Jun;13(6):1037-47. doi: 10.1038/sj.cdd.4401926. Cell Death Differ. 2006. PMID: 16601749 - Role of the p63-FoxN1 regulatory axis in thymic epithelial cell homeostasis during aging.
Burnley P, Rahman M, Wang H, Zhang Z, Sun X, Zhuge Q, Su DM. Burnley P, et al. Cell Death Dis. 2013 Nov 21;4(11):e932. doi: 10.1038/cddis.2013.460. Cell Death Dis. 2013. PMID: 24263106 Free PMC article. - Differential expression of p63 isoforms in normal tissues and neoplastic cells.
Nylander K, Vojtesek B, Nenutil R, Lindgren B, Roos G, Zhanxiang W, Sjöström B, Dahlqvist A, Coates PJ. Nylander K, et al. J Pathol. 2002 Dec;198(4):417-27. doi: 10.1002/path.1231. J Pathol. 2002. PMID: 12434410 - p63 in tooth development.
Rufini A, Barlattani A, Docimo R, Velletri T, Niklison-Chirou MV, Agostini M, Melino G. Rufini A, et al. Biochem Pharmacol. 2011 Nov 15;82(10):1256-61. doi: 10.1016/j.bcp.2011.07.068. Epub 2011 Jul 20. Biochem Pharmacol. 2011. PMID: 21787761 Review. - Dynamic life of a skin keratinocyte: an intimate tryst with the master regulator p63.
Romano RA, Sinha S. Romano RA, et al. Indian J Exp Biol. 2011 Oct;49(10):721-31. Indian J Exp Biol. 2011. PMID: 22013738 Review.
Cited by
- Lyophilized powder of mesenchymal stem cell supernatant attenuates acute lung injury through the IL-6-p-STAT3-p63-JAG2 pathway.
Peng W, Chang M, Wu Y, Zhu W, Tong L, Zhang G, Wang Q, Liu J, Zhu X, Cheng T, Li Y, Chen X, Weng D, Liu S, Zhang H, Su Y, Zhou J, Li H, Song Y. Peng W, et al. Stem Cell Res Ther. 2021 Mar 29;12(1):216. doi: 10.1186/s13287-021-02276-y. Stem Cell Res Ther. 2021. PMID: 33781349 Free PMC article. - Novel mutation in TP63 associated with ectrodactyly ectodermal dysplasia and clefting syndrome and T cell lymphopenia.
Giampietro PF, Baker MW, Basehore MJ, Jones JR, Seroogy CM. Giampietro PF, et al. Am J Med Genet A. 2013 Jun;161A(6):1432-5. doi: 10.1002/ajmg.a.35885. Epub 2013 Apr 23. Am J Med Genet A. 2013. PMID: 23613309 Free PMC article. - HPV16 E5 and KGFR/FGFR2b interplay in differentiating epithelial cells.
Purpura V, Belleudi F, Caputo S, Torrisi MR. Purpura V, et al. Oncotarget. 2013 Feb;4(2):192-205. doi: 10.18632/oncotarget.803. Oncotarget. 2013. PMID: 23545251 Free PMC article. - A Rare Case of _TP63_-Associated Lymphopenia Revealed by Newborn Screening Using TREC.
Marakhonov A, Serebryakova E, Mukhina A, Vechkasova A, Prokhorov N, Efimova I, Balinova N, Lobenskaya A, Vasilyeva T, Zabnenkova V, Ryzhkova O, Rodina Y, Pershin D, Soloveva N, Fomenko A, Saydaeva D, Ibisheva A, Irbaieva T, Koroteev A, Zinchenko R, Voronin S, Shcherbina A, Kutsev S. Marakhonov A, et al. Int J Mol Sci. 2024 Oct 9;25(19):10844. doi: 10.3390/ijms251910844. Int J Mol Sci. 2024. PMID: 39409174 Free PMC article. - Allele-specific silencing of EEC p63 mutant R304W restores p63 transcriptional activity.
Novelli F, Lena AM, Panatta E, Nasser W, Shalom-Feuerstein R, Candi E, Melino G. Novelli F, et al. Cell Death Dis. 2016 May 19;7(5):e2227. doi: 10.1038/cddis.2016.118. Cell Death Dis. 2016. PMID: 27195674 Free PMC article.
References
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F. Mol Cell. 1998;2:305–316. - PubMed
- Yang A, Walker N, Bronson R, Kaghad M, Oosterwegel M, Bonnin J, Vagner C, Bonnet H, Dikkes P, Sharpe A, et al. Nature. 2000;404:99–103. - PubMed
- Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, et al. Nature. 1999;398:714–718. - PubMed
- Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. Nature. 1999;398:708–713. - PubMed
- Yang A, McKeon F. Nat Rev Mol Cell Biol. 2000;1:199–207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous