Integrin-mediated host cell invasion by type 1-piliated uropathogenic Escherichia coli - PubMed (original) (raw)
Integrin-mediated host cell invasion by type 1-piliated uropathogenic Escherichia coli
Danelle S Eto et al. PLoS Pathog. 2007 Jul.
Abstract
Uropathogenic Escherichia coli (UPEC), the primary causative agent of urinary tract infections, typically express filamentous adhesive organelles called type 1 pili that mediate both bacterial attachment to and invasion of bladder urothelial cells. Several host proteins have previously been identified as receptors for type 1 pili, but none have been conclusively shown to promote UPEC entry into host bladder cells. Using overlay assays with FimH, the purified type 1 pilus adhesin, and mass spectroscopy, we have identified beta1 and alpha3 integrins as key host receptors for UPEC. FimH recognizes N-linked oligosaccharides on these receptors, which are expressed throughout the urothelium. In a bladder cell culture system, beta1 and alpha3 integrin receptors co-localize with invading type 1-piliated bacteria and F-actin. FimH-mediated bacterial invasion of host bladder cells is inhibited by beta1 and alpha3 integrin-specific antibodies and by disruption of the beta1 integrin gene in the GD25 fibroblast cell line. Phosphorylation site mutations within the cytoplasmic tail of beta1 integrin that alter integrin signaling also variably affect UPEC entry into host cells, by either attenuating or boosting invasion frequencies. Furthermore, focal adhesion and Src family kinases, which propagate integrin-linked signaling and downstream cytoskeletal rearrangements, are shown to be required for FimH-dependent bacterial invasion of target host cells. Cumulatively, these results indicate that beta1 and alpha3 integrins are functionally important receptors for type 1 pili-expressing bacteria within the urinary tract and possibly at other sites within the host.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
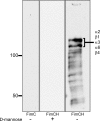
Figure 1. Identification of Potential FimH Receptors
Membrane-associated proteins isolated from 5637 bladder epithelial cells were resolved by SDS-PAGE and transferred to PVDF membrane. Protein bands bound by purified recombinant FimC6XHisFLAG alone (left) or FimC6XHisFLAG–FimH complexes in the presence (middle) and absence (right) of 2.5% D-mannose were detected by chemiluminescence in overlay assays using anti-FLAG antibody. The two most prominent bands bound by the FimC6XHisFLAG–FimH complexes were found by mass spectroscopy analysis to be comprised of α2, β1, α3, α6, and β4 integrins. Molecular weight standards are indicated on the left.
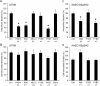
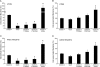
Figure 2. Anti–β1 and Anti–α3 Integrin Antibodies Inhibit Host Cell Invasion by Type 1–Piliated E. coli
5637 bladder epithelial cell monolayers were treated with 1.5 μg/well of the indicated monoclonal antibodies for 30 min prior to infection with the UPEC cystitis isolate UTI89 or AAEC185/pSH2, a type 1 pili–expressing recombinant K-12 strain. Shown are (A and C) intracellular bacterial titers, determined using gentamicin protection assays, and (B and D) total cell-associated bacteria (including both intra- and extracellular bacteria). Data are expressed as the means ± standard error of the mean of at least three independent experiments carried out in triplicate. * p < 0.001, versus untreated control (Ctrl) values, as determined by Student's _t_-test.
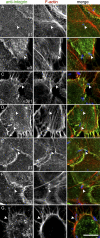
Figure 3. Localization of β1 and α3 Integrins with Type 1 Pili–Expressing E. coli
5637 bladder cells were infected for 30 min with (A–D) UTI89 or (E–G) AAEC185/pSH2 prior to fixation and processing for immunofluorescent confocal microscopy. Samples were stained using antibodies specific for the following individual integrin subunits: (A and E) β1, (B and F) α3, or (C, D and G) heterodimeric α3β1 integrin complexes (green in the merged color images). F-actin (red) was visualized using Alexa568-conjugated phalloidin, while bacteria (blue) were detected using anti–E. coli antibody. For clarity, the merged color image in each row is accompanied by images showing only corresponding single channel signals from integrin or F-actin staining. Arrowheads denote the location of an individual bacterium in each set of images. Based on optical sectioning, the highlighted bacteria in (A–C) and (E–G) were localized at or very near the host cell surface, while the bacteria in (D) have already completely penetrated the target host cell. Scale bar = 10 μm.
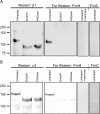
Figure 4. Glycosidase Treatment Abrogates In Vitro Binding of FimH to β1 and α3 Integrins
(A) β1 and (B) α3 integrins were immunoprecipitated from 5637 cells that had been transiently transfected with plasmids for overexpression of recombinant human β1 or α3 integrins. The immunoprecipitated proteins were treated ± glycosidases (endoglycosidase Hf [EndoHf] or Peptide: N-glycosidase F [PNGase]) prior to SDS-PAGE and transfer to PVDF membranes. Blots were probed with either (A) anti–β1 or (B) anti–α3 integrin antibodies (Westerns), revealing expected shifts in the electrophoretic mobility of both integrin subunits following glycosidase treatments. For each set of samples, duplicate blots were overlaid with purified recombinant FimC6XHisFLAG–FimH complexes and probed with anti-FLAG tag antibody (Far Westerns). As additional controls, blots containing untreated β1 or α3 integrins were also incubated with either FimC6XHisFLAG–FimH complexes plus 2.5% D-mannose or with FimC6XHisFLAG alone. Molecular weight standards are indicated on the left.
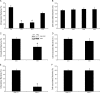
Figure 5. β1 Integrin Cytoplasmic Tail Mutants Differentially Affect FimH-Mediated Bacterial Invasion of Host Cells
β1 integrin–null GD25 cells, as well as GD25 derivatives that constitutively express wild-type or the indicated mutant forms of β1 integrin, were infected with (A and B) UTI89 or (C and D) AAEC185/pSH2. Levels of intracellular bacteria (A and C) were normalized among the different host cell lines by dividing the numbers of intracellular, gentamicin-protected bacteria by the number of total cell-associated bacteria (B and D). Data are expressed relative to results from GD25-β1A control cells and represent the means ± standard error of the mean of at least five independent experiments performed in triplicate. * p < 0.001, versus values from control GD25-β1A cells, as determined by Student's _t_-test.
Figure 6. FimH-Mediated Bacterial Invasion of Host Cells Requires Src Family Kinases and FAK
(A and B) 5637 cells were treated with 20 μg/ml of the Src family kinase inhibitors PP1 or PP2 or with an inactive analog, PP3, for 1 h prior to infection with UTI89. Levels of intracellular bacteria as determined by gentamicin protection assays (A) or total cell-associated bacteria (B) are expressed relative to results from cells treated with carrier (DMSO) alone. (C and D) Alternately, 5637 cells were transfected with either scrambled control siRNA or siRNA with specificity against FAK. Cells were infected with UTI89 at 72 h after siRNA transfection and intracellular (C) and total cell-associated (D) bacterial counts were determined. FAK knockdown was verified by Western blot analysis using antibody specific for total FAK ([C], inset). Blots were also probed using anti-actin antibody as a protein loading control. (E and F) Intracellular (E) and total cell-associated (F) bacteria were also quantified in assays using FAK−/− and control FAK+/+ mouse embryo fibroblasts. Intracellular bacterial levels were normalized in all assays by dividing the numbers of intracellular, gentamicin-protected bacteria by the number of total cell-associated bacteria. Data are expressed relative to results from the indicated control samples and represent the means ± standard error of the mean of three independent experiments performed in triplicate. * p < 0.001, versus values from control samples, as determined by Student's _t_-test.
Similar articles
- Type 1 pilus-mediated bacterial invasion of bladder epithelial cells.
Martinez JJ, Mulvey MA, Schilling JD, Pinkner JS, Hultgren SJ. Martinez JJ, et al. EMBO J. 2000 Jun 15;19(12):2803-12. doi: 10.1093/emboj/19.12.2803. EMBO J. 2000. PMID: 10856226 Free PMC article. - Requirement of Rho-family GTPases in the invasion of Type 1-piliated uropathogenic Escherichia coli.
Martinez JJ, Hultgren SJ. Martinez JJ, et al. Cell Microbiol. 2002 Jan;4(1):19-28. doi: 10.1046/j.1462-5822.2002.00166.x. Cell Microbiol. 2002. PMID: 11856170 - Uropathogenic Escherichia coli induces extrinsic and intrinsic cascades to initiate urothelial apoptosis.
Klumpp DJ, Rycyk MT, Chen MC, Thumbikat P, Sengupta S, Schaeffer AJ. Klumpp DJ, et al. Infect Immun. 2006 Sep;74(9):5106-13. doi: 10.1128/IAI.00376-06. Infect Immun. 2006. PMID: 16926402 Free PMC article. - Covert operations of uropathogenic Escherichia coli within the urinary tract.
Bower JM, Eto DS, Mulvey MA. Bower JM, et al. Traffic. 2005 Jan;6(1):18-31. doi: 10.1111/j.1600-0854.2004.00251.x. Traffic. 2005. PMID: 15569242 Free PMC article. Review. - Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses.
Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ. Mulvey MA, et al. Proc Natl Acad Sci U S A. 2000 Aug 1;97(16):8829-35. doi: 10.1073/pnas.97.16.8829. Proc Natl Acad Sci U S A. 2000. PMID: 10922042 Free PMC article. Review.
Cited by
- Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era.
Kostakioti M, Hadjifrangiskou M, Hultgren SJ. Kostakioti M, et al. Cold Spring Harb Perspect Med. 2013 Apr 1;3(4):a010306. doi: 10.1101/cshperspect.a010306. Cold Spring Harb Perspect Med. 2013. PMID: 23545571 Free PMC article. Review. - Polysaccharide capsule and sialic acid-mediated regulation promote biofilm-like intracellular bacterial communities during cystitis.
Anderson GG, Goller CC, Justice S, Hultgren SJ, Seed PC. Anderson GG, et al. Infect Immun. 2010 Mar;78(3):963-75. doi: 10.1128/IAI.00925-09. Epub 2010 Jan 19. Infect Immun. 2010. PMID: 20086090 Free PMC article. - Molecular mechanisms of Escherichia coli pathogenicity.
Croxen MA, Finlay BB. Croxen MA, et al. Nat Rev Microbiol. 2010 Jan;8(1):26-38. doi: 10.1038/nrmicro2265. Nat Rev Microbiol. 2010. PMID: 19966814 - Dictamnine Inhibits the Adhesion to and Invasion of Uropathogenic Escherichia Coli (UPEC) to Urothelial Cells.
Yang W, Liu P, Chen Y, Lv Q, Wang Z, Huang W, Jiang H, Zheng Y, Jiang Y, Sun L. Yang W, et al. Molecules. 2022 Jan 2;27(1):272. doi: 10.3390/molecules27010272. Molecules. 2022. PMID: 35011504 Free PMC article. - Biomimetic delivery strategies at the urothelium: targeted cytoinvasion in bladder cancer cells via lectin bioconjugates.
Neutsch L, Eggenreich B, Herwig E, Marchetti-Deschmann M, Allmaier G, Gabor F, Wirth M. Neutsch L, et al. Pharm Res. 2014 Mar;31(3):819-32. doi: 10.1007/s11095-013-1204-3. Epub 2013 Dec 24. Pharm Res. 2014. PMID: 24366662
References
- Foxman B, Brown P. Epidemiology of urinary tract infections: Transmission and risk factors, incidence, and costs. Infect Dis Clin North Am. 2003;17:227–241. - PubMed
- Foxman B, Barlow R, D'Arcy H, Gillespie B, Sobel JD. Urinary tract infection: Self-reported incidence and associated costs. Ann Epidemiol. 2000;10:509–515. - PubMed
- Ronald A. The etiology of urinary tract infection: Traditional and emerging pathogens. Am J Med. 2002;113(Suppl 1A):14S–19S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK068585/DK/NIDDK NIH HHS/United States
- R21 DK069526/DK/NIDDK NIH HHS/United States
- R01 DK068585-04/DK/NIDDK NIH HHS/United States
- T32 AI055434/AI/NIAID NIH HHS/United States
- T32 AI055434-01A1/AI/NIAID NIH HHS/United States
- DK069526/DK/NIDDK NIH HHS/United States
- R01 DK068585/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous