Inhibition of proliferation by PERK regulates mammary acinar morphogenesis and tumor formation - PubMed (original) (raw)
Inhibition of proliferation by PERK regulates mammary acinar morphogenesis and tumor formation
Sharon J Sequeira et al. PLoS One. 2007.
Abstract
Endoplasmic reticulum (ER) stress signaling can be mediated by the ER kinase PERK, which phosphorylates its substrate eIF2alpha. This in turn, results in translational repression and the activation of downstream programs that can limit cell growth through cell cycle arrest and/or apoptosis. These responses can also be initiated by perturbations in cell adhesion. Thus, we hypothesized that adhesion-dependent regulation of PERK signaling might determine cell fate. We tested this hypothesis in a model of mammary acini development, a morphogenetic process regulated in part by adhesion signaling. Here we report a novel role for PERK in limiting MCF10A mammary epithelial cell proliferation during acinar morphogenesis in 3D Matrigel culture as well as in preventing mammary tumor formation in vivo. We show that loss of adhesion to a suitable substratum induces PERK-dependent phosphorylation of eIF2alpha and selective upregulation of ATF4 and GADD153. Further, inhibition of endogenous PERK signaling during acinar morphogenesis, using two dominant-negative PERK mutants (PERK-DeltaC or PERK-K618A), does not affect apoptosis but results instead in hyper-proliferative and enlarged lumen-filled acini, devoid of proper architecture. This phenotype correlated with an adhesion-dependent increase in translation initiation, Ki67 staining and upregulation of Laminin-5, ErbB1 and ErbB2 expression. More importantly, the MCF10A cells expressing PERKDeltaC, but not a vector control, were tumorigenic in vivo upon orthotopic implantation in denuded mouse mammary fat pads. Our results reveal that the PERK pathway is responsive to adhesion-regulated signals and that it is essential for proper acinar morphogenesis and in preventing mammary tumor formation. The possibility that deficiencies in PERK signaling could lead to hyperproliferation of the mammary epithelium and increase the likelihood of tumor formation, is of significance to the understanding of breast cancer.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
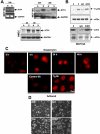
Figure 1. Suspension Induces Phosphorylation of eIF2α and Translation Repression in Mammary and Kidney Epithelial Cells.
(A) Whole cell lysates from MCF10A (upper left), HEK293T (upper right) and primary HMEC (lower panels) cells grown either in adhered (A) or suspended conditions (S) as described in the methods section for the indicated time points, were immunoblotted for p-eIF2α and total eIF2α levels. Adhered MCF10A or HEK293T cells treated with 2 mM DTT or 5 µg/ml tunicamycin (Tn) respectively, were used as positive controls. (B) Quantification of the rate of DNA synthesis using a BrdU incorporation assay and flow cytometry to measure the percentage of BrdU-positive cells (filled diamonds) at different time points in suspension. The percentage of apoptotic cells was measured using propidium iodide staining and flow cytometry to identify the sub-G0 apoptotic fraction for adhered (dashed line) or suspended (dotted line) MCF10A cells for different time points. Data points show the mean±SD for BrdU–positive cells in each sample as a percentage of the total. (C) Autoradiogram of [35S] Met/Cys incorporation (right panel) into newly synthesized proteins in MCF10A cells adhered or suspended for 24 hrs (two independent samples). Coomassie Blue staining of an identical gel (left panel) shows equal protein loading. (D) Polysome profiles from 24 hr adhered (left) and suspended (right) MCF10A cells showing an increase and decrease in the monosome and polysome peaks, respectively in suspended cells. Absorbance at 254 nm (Y-axis, RNA concentration) was plotted against migration in the sucrose gradient (X-axis, bottom to top). Total RNA was isolated from individual fractions to visualize the 18S and 28S rRNAs by ethidium bromide staining.
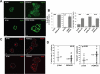
Figure 2. ATF4 Protein Levels Are Strongly Upregulated During Suspension Conditions.
(A) RT-PCR analysis of ATF4 or GAPDH (as loading control) mRNA levels, in adhered (A) or suspended (S) MCF10A cells, (left panels); ATF4 protein levels or GAPDH (as loading control) in MCF10A (right panels) and primary HMEC cells (bottom panels) as detected by Western blotting. (B) Immunoblot showing increased levels of p-eIF2α (upper panels) or ATF4 (lower panels) protein comparable to that induced by suspension, in MCF10A cells pretreated with AIIB2, a β1-integrin function-blocking Ab (10 µg/ml). Control cells were treated with an isotype-matched IgG (10 µg/ml). Total eIF2α was used as loading control. (C) Immunofluorescent staining for ATF4 (red) in MCF10A cells immediately after detachment (0 hrs) and placed in suspension for the indicated time points or treated with thapsigargin (4 µM) for 6 hrs before fixing on poly-lysine-coated coverslips to facilitate detection. (D) Photomicrographs of MCF10A cells plated on Laminin-1 coated tissue culture dishes, pre-incubated with AIIB2 (10 µg/ml) or a control IgG antibody (10 µg/ml) prior to plating or placed in suspension on agar-coated dishes in the presence of 0.5% methylcellulose in media containing 1% serum.
Figure 3. GADD153 mRNA and Protein Levels Are Strongly Upregulated During Suspension Conditions.
(A) RT-PCR analysis of GADD153 mRNA levels in MCF10A (left panel) and HEK293T (right panel) cells at different time points in either adhered (A) or suspended (S) conditions. Adhered MCF10A cells treated with 2mM DTT for 4h were used as positive control and GAPDH was used as a loading control. (B) MCF10A cells were transiently transfected with a GADD153 promoter-driven-EGFP reporter plasmid and EGFP fluorescence was analyzed 48 h post-transfection by FACS; total events captured: 2×104. The graph shows the number of GFP-positive events in FLH2>10 (mean±SD). (C) Western blot for GADD153 protein in adhered (A) or suspended (S) MCF10A cells. (D and E) Immunofluorescence (D) and FACS (E) analysis of GADD153 (red) expression in MCF10A cells following growth in adhered or suspension conditions for the indicated times. Secondary antibody was used as negative control in E. (F) MCF10A (top) and HEK293T (lower) cells were transiently co-transfected with the GADD153-EGFP reporter plasmid and either a full-length Flag-tagged GADD34 plasmid or an empty vector as control for 24 hrs before being detached and left to reattach or put into suspension for an additional 48 hrs before FACS analysis. GFP fluorescence was analyzed 48 h post transfection by FACS where a total 2×104 events were captured. The graphs show the number of GFP positive events in FLH2>10 or the mean fluorescence intensity (MFI) in the PMT2-FITC channel (mean±SD). (G) RT-PCR for XBP-1 splicing (top panel) in MCF10A cells at different time points either adhered (A) or suspended (S). Adhered MCF10A cells treated with 2mM DTT for 4 hrs was used as positive control and GAPDH, shown in (A) was used as a loading control. Lower panels show RT-PCR for BiP, Hsp47 and Erp72/PDI chaperone mRNA levels in adhered (Adh) or suspended (Sus) MCF10A cells. GAPDH was used as a loading control.
Figure 4. PERK is the Major Upstream Kinase Mediating Phosphorylation of eIF2α in Suspension.
(A and B) Immunoblot for phospho- and total GCN2 and PKR in MCF10A cells in adhered or suspension conditions for 24 and 48 hrs. Note that only a faint phospho-protein band for phospho-GCN2 was observed at the 220 kD position but in adhered conditions. GAPDH was used to show equal loading. (C) IB for phospho-(top blot) and total- (bottom blot) eIF2α in PERK wild-type and PERK-/-MEFs grown in adhesion (A) or in suspension (S) for 24 hrs. (D) IB for phospho- and total PERK in MCF10A cells grown adhered (A) or in suspension (S) for 24 hrs. (E) IB for phospho- PERK and myc in HEK293T cells transiently transfected with empty vector control or mouse WT-myc tagged-PERK and placed in adhered (A) or suspended (S) conditions for 24 hrs. GAPDH serves as loading control. (F) IB for Myc-PERKΔC expression in parental and in MCF10A cells stably expressing either pBabepuro-myc-PERKΔC or a vector control pBabepuro-β-galactosidase construct. (G) IB for phospho- and total eIF2α and ATF4 expression in β-Gal or myc-PERKΔC expressing cells either adhered or suspended for 24 and 48 hrs. GAPDH was used as loading control. (H) IB for Myc, phospho and total eIF2α and GAPDH in MCF10A cells stably expressing β-Gal (vector control) or a pBabeneo-PERK-K618A (kinase dead) mutant. (I) Cells obtained in (H) were used in a suspension vs. adhesion assay for 24 hrs and ATF4 and GADD153 expression was detected through IB in the cell lysates. GAPDH was used as a loading control.
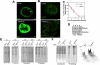
Figure 5. PERK is Required to Maintain Normal Mammary Acini Growth and Morphogenesis through Regulation of Cell Proliferation.
(A) Representative images through the equatorial cross sections of acini from myc-PERKΔC, myc-PERK-K618A or β-gal expressing MCF10A cells on Day 8 of culture in 3D Matrigel, using LSCM. Nuclei are stained green using CyQuant dye. Note the increase in acinar size, luminal filling and the multi-lobular nature of the PERKΔC structures as compared to the vector control acini. PERK-K618A expressing acini were also significantly larger and always showed luminal cells, but were not frequently multi-lobulated. Scale bars = 20 µm. (B) Acinar size was measured on different days in Matrigel for β-Gal and PERKΔC or PERK-K618A cells, using SPOT™ software to measure two perpendicular diameters per acinus and calculate acinar volume considering an ellipsoid structure; volume for 50–200 acini were quantitated per time point, shown as percentage of total for the given size range, Mean±SD. (C) Confocal images for β-Gal and PERKΔC cells immunostained for cleaved (*) caspase-3 (red, top panels) or Ki-67 (red, bottom panels) on Day 8 in Matrigel. Acini are outlined with white dotted lines to delineate the normal β-Gal vs. disorganized large PERKΔC acini, which shows Ki-67 positively stained cells in the lumen. Scale bars = 20 µm. (D) Graphs showing distribution of the percentage of cells per acinus that stained positively for Ki-67 or cleaved caspase-3 in β-Gal and PERKΔC cells on Day 8 in Matrigel; between 50–100 acini were scored and statistical significance was determined by the t test for independent samples with P<0.001 defined as statistically significant. #N.S-not significant.
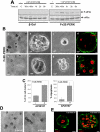
Figure 6. Immunofluorescence for ESA and E-cadherin In β-Gal Control and PERKΔC Acini.
(A) Representative confocal images of cross sections through β-Gal and PERKΔC acini immunostained for the cell surface glycoprotein, ESA (epithelial specific antigen, green), on Day 8 in Matrigel. Note the presence of ESA in cells occupying the luminal space of PERKΔC acini (bottom) as compared to proper basolateral localization of ESA in the β-Gal control acini (top). (B) Representative confocal images of β-Gal and PERKΔC acini co-stained for E-cadherin (green) and Ki-67 (red), on Day 8 in Matrigel. Note the presence of strong E-cadherin staining in the cell-cell junctions of luminal cells in PERKΔC acini that also stain positive for Ki-67 while β-Gal control acini show little to no Ki-67 staining in luminal cells and the expected apicolateral staining for E-cadherin in the outer rim of cells. Scale bars = 10 µm. (C) Proliferation assay using BrdU incorporation and flow cytometry to measure the percentage of BrdU-positive β-Gal or PERKΔC cells at different time points in suspension, mean±SD. (D) Immunoblot showing an increase in BimEL levels in suspension but no significant change between β-Gal and PERKΔC cells, GAPDH serves as loading control. (E) Autoradiogram of [35S] Met/Cys incorporation into newly synthesized proteins in β-Gal or PERKΔC cells in full (5%) serum media placed in adhered or suspended growth conditions for 8 hrs, 12 hrs or 24 hrs, showing complete attenuation of protein synthesis in suspension. Coomassie Blue staining of identical gels (left panels) shows protein loading. (F) Autoradiogram of [35S] Met/Cys incorporation into newly synthesized proteins in adhered β-Gal or PERKΔC cells placed in low (1%) serum media, treated with or without DTT (2 mM for 1hr), showing increased protein synthesis in PERKΔC cells. Coomassie Blue staining of an identical gel (left panel) shows equal protein loading. Densitometric analysis and surface plotting of the bands illustrates the increase in intensity in the PERKΔC cells.
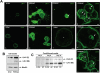
Figure 7. Unscheduled Activation of PERK Restricts Acinar Growth and Promotes Apoptosis in 3D Matrigel.
(A) Time-dependent increase in phosphorylation of eIF2α in MCF10A cells expressing an Fv2E-ΔNPERK construct, upon treatment with the dimerizing drug, AP20187 (2 nM). AP20187 has no effect on P-eIF2α levels in β-Gal cells. Total eIF2α was used as loading control. (B) Photomicrographs of Fv2E-ΔNPERK cells in 3D Matrigel treated with 2 nM AP20187 or equal volume of ethanol as control, added every 24 hrs from Day 4 up to Day 6 of morphogenesis; representative phase-contrast images depict the effect of forced PERK activation on acini development; (B-a and B-e) A×10 magnification image of several developing acini; (B-b) Normal acinus, (B-c) 2 cell cluster, (B-f) 4 cell cluster, (B-g) 4 cell cluster containing apoptotic cells. (B-d and h) Confocal images through the equatorial region of Fv2E-ΔNPERK cells in 3D Matrigel immunostained for active caspase-3 (red) or LN-5 (green) with (B-h) and without (B-d) treatment with 2nM AP20187 every 24 hrs, (B-h) cell cluster where the majority of cells have entered apoptosis. (C) Quantitation of phase contrast images of Fv2E-ΔNPERK cells on Day 6, treated every 24 hrs with or without 2nM AP20187. Over 400 acini were visually scored for the presence of apoptotic or growth arrested 2–4 celled acini and calculated as a percentage of the total number of acini; graph shows mean±SD. (D) Photomicrographs of β-Gal vector control cells treated with 2nM AP20187 or with equal volume EtOH as control, every 24 hrs from Day 4 up to Day 6 in Matrigel. Note that AP20187 treatment caused no noticeable changes in acini size or morphology, consistent with the absence of modulation of P-eIF2α levels in the same cells (A). (E) Confocal images showing Fv2E-ΔNPERK cells treated with (+AP) or without (-AP) AP20187 every 12 hrs stained for LN-5 (green) to delineate the acini and active caspase-3 (red). Note that a majority of cells even in large acini can be pushed into apoptosis by strong activation of PERK signaling. Scale bars = 10 µm.
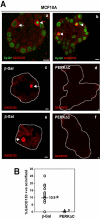
Figure 8. GADD153 Upregulation During Acini Development in 3D Cultures is PERK-dependent.
(Aa and Ab) Representative confocal images through the equatorial section of Day10 MCF10A acini depicts strong GADD153-positive staining (red) in cells in the luminal space (arrowheads) that also display condensed nuclei, co-stained with CyQuant (green) as well as in some cases, in cells that localize to the outer basal layer of cells (arrow) that do not have condensed nuclei. (Ac-f) Representative images of GADD153 staining (red) in β-Gal (Ac and Ae) and PERKΔC (Ad and Af), cells on Day 8 in Matrigel. Scale bars = 10 µm. (B) Graph showing the distribution and median of GADD153- positive acini/field; 21 fields adding up to a total of 215 acini were scored.* P<0.001 determined by t test for independent samples.
Figure 9. PERK Inhibition Results in Increased Laminin-5 Production, Secretion and Deposition.
(A) Confocal images showing the equatorial cross sections through β-Gal (A-a, A-b and A-f) and PERKΔC (A-c to A-e and A-g to A-i) acini stained for Laminin-5 (green), on Day 8 in 3D. Note the increased staining and disorganized Laminin-5 deposition in PERKΔC cells as compared to proper basal localization in the β-Gal control cells. Panels A-e and A-g through A-i (arrows) show details of intra-acinar deposition of Laminin-5 within and around cells in the filled lumen. Scale bars = 10 µm; A-h is a magnified image of an acinus shown in A-g. (B) Western blot analysis from β-Gal and PERKΔC cell lysates shows the precursor (P) and mature (M) forms of LN-5. BimEL was used as loading control. (C) Western blot for Laminin-5 secreted into the conditioned media by β-Gal and PERKΔC cells grown in 3D-Matrigel. For conditioned media, samples corresponding to days 4 and 8, the supernatant was collected after 96 hrs of 3D culture. For the conditioned media sample corresponding to day 10, the supernatant was collected after 48 hrs of 3D culture. ΔC = MCF10A-PERKΔC cells. Note that while in cell lysates (B) the precursor is predominantly detected, in the conditioned media there is stronger detection of the mature form of the Laminin-5-γ2 subunit.
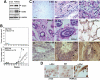
Figure 10. PERK Inhibition in MCF10A Cells Favors Mammary Tumor Formation.
(A) Western blot for ErbB1 (EGFR) and ErbB2 (Her2) levels in adhered β-Gal or PERKΔC cells show increased levels when PERK is inhibited. GAPDH was used as loading control. (B) β-Gal or PERKΔC cells were injected orthotopically into the contralateral abdominal mammary fat pads of 3 week-old female nude mice (see methods for details). Post-implantation mice were monitored biweekly for tumor take and when detected tumor diameters were measured and the volume was calculated and plotted as described in methods. Note that none of the mice implanted with β-Gal-MCF10A cells developed tumor nodules. (C) Histology of mammary glands and tumors in mice implanted with β-Gal and PERKΔC expressing MCF10A cells. (C-a) H&E staining of a cleared mammary fat pad inoculated with β-Gal cells. Only adipose tissue and occasional stromal cells that were negative for β-galactosidase activity was observed. (C-b) H&E staining of a PERKΔC tumor lesion. Note the disorganization of the fibrotic-epithelial tissue. Arrows depict the presence of epithelial cells forming acinar or duct-like structures within the PERKΔC tumor lesion. (C-c) Higher magnification of intratumoral accumulations of cells without a defined architecture but comprised of a mixture of epithelial (larger nuclei and pink cytoplasm) and inflammatory cells (smaller darkly stained nuclei). (C-d and e) PERKΔC cells form acini-like structures with an empty lumen or show hyperplastic growth and a repopulated lumen (C-e, top left and right insets). (C-f) Anti-Myc (αMyc) staining in control β-Gal injected mammary fat pads. Note that only a light background signal is observed in adipocytes. (C-g) Histological section of a PERKΔC acinus-like structure stained with a non-specific IgG (arrow denotes the lack of staining in these epithelial cells) or with anti-Myc 9E10 mAb (C-h and C-i); arrows denote the brown staining generated by Myc-tag detection. (D) β-Gal (left panel) or PERKΔC (middle and right panels) cells grown on 2D coverslips and fixed and stained with a non-specific IgG (IgG) or with an anti-Myc 9E10 mAb (α-Myc). The Myc staining was characteristic of intracellular cistern distribution.
Similar articles
- Inhibition of eIF2alpha dephosphorylation inhibits ErbB2-induced deregulation of mammary acinar morphogenesis.
Sequeira SJ, Wen HC, Avivar-Valderas A, Farias EF, Aguirre-Ghiso JA. Sequeira SJ, et al. BMC Cell Biol. 2009 Sep 15;10:64. doi: 10.1186/1471-2121-10-64. BMC Cell Biol. 2009. PMID: 19754954 Free PMC article. - Pollen Typhae Total Flavone Inhibits Endoplasmic Reticulum Stress-Induced Apoptosis in Human Aortic-Vascular Smooth Muscle Cells through Down-Regulating PERK-eIF2α-ATF4-CHOP Pathway.
Chen MT, Huang RL, Ou LJ, Chen YN, Men L, Chang X, Wang L, Yang YZ, Zhang Z. Chen MT, et al. Chin J Integr Med. 2019 Aug;25(8):604-612. doi: 10.1007/s11655-019-3052-4. Epub 2019 Feb 1. Chin J Integr Med. 2019. PMID: 30707413 - The Role of the PERK/eIF2α/ATF4/CHOP Signaling Pathway in Tumor Progression During Endoplasmic Reticulum Stress.
Rozpedek W, Pytel D, Mucha B, Leszczynska H, Diehl JA, Majsterek I. Rozpedek W, et al. Curr Mol Med. 2016;16(6):533-44. doi: 10.2174/1566524016666160523143937. Curr Mol Med. 2016. PMID: 27211800 Free PMC article. Review. - Coping with stress: eIF2 kinases and translational control.
Wek RC, Jiang HY, Anthony TG. Wek RC, et al. Biochem Soc Trans. 2006 Feb;34(Pt 1):7-11. doi: 10.1042/BST20060007. Biochem Soc Trans. 2006. PMID: 16246168 Review.
Cited by
- Paradoxical effects of DNA tumor virus oncogenes on epithelium-derived tumor cell fate during tumor progression and chemotherapy response.
He J, Liu L, Tang F, Zhou Y, Liu H, Lu C, Feng D, Zhu H, Mao Y, Li Z, Zhang L, Duan Y, Xiao Z, Zeng M, Weng L, Sun LQ. He J, et al. Signal Transduct Target Ther. 2021 Nov 26;6(1):408. doi: 10.1038/s41392-021-00787-x. Signal Transduct Target Ther. 2021. PMID: 34836940 Free PMC article. - Tumor cell dormancy.
Gomis RR, Gawrzak S. Gomis RR, et al. Mol Oncol. 2017 Jan;11(1):62-78. doi: 10.1016/j.molonc.2016.09.009. Epub 2016 Oct 7. Mol Oncol. 2017. PMID: 28017284 Free PMC article. Review. - Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration.
Bravo R, Parra V, Gatica D, Rodriguez AE, Torrealba N, Paredes F, Wang ZV, Zorzano A, Hill JA, Jaimovich E, Quest AF, Lavandero S. Bravo R, et al. Int Rev Cell Mol Biol. 2013;301:215-90. doi: 10.1016/B978-0-12-407704-1.00005-1. Int Rev Cell Mol Biol. 2013. PMID: 23317820 Free PMC article. Review. - OSU-03012 sensitizes breast cancers to lapatinib-induced cell killing: a role for Nck1 but not Nck2.
West NW, Garcia-Vargas A, Chalfant CE, Park MA. West NW, et al. BMC Cancer. 2013 May 24;13:256. doi: 10.1186/1471-2407-13-256. BMC Cancer. 2013. PMID: 23706161 Free PMC article. - Epithelial Xbp1 is required for cellular proliferation and differentiation during mammary gland development.
Hasegawa D, Calvo V, Avivar-Valderas A, Lade A, Chou HI, Lee YA, Farias EF, Aguirre-Ghiso JA, Friedman SL. Hasegawa D, et al. Mol Cell Biol. 2015 May;35(9):1543-56. doi: 10.1128/MCB.00136-15. Epub 2015 Feb 23. Mol Cell Biol. 2015. PMID: 25713103 Free PMC article.
References
- Harris J, Lippman M, Morrow M, Osborne C. Diseases of the breast. 1999
- Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, et al. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40. - PubMed
- Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, et al. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol. 2003;5:733–740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA119018/CA/NCI NIH HHS/United States
- R01 CA109182-02/CA/NCI NIH HHS/United States
- R01 CA109182-01A1/CA/NCI NIH HHS/United States
- CA109182/CA/NCI NIH HHS/United States
- R01 CA119018/CA/NCI NIH HHS/United States
- R01 CA109182/CA/NCI NIH HHS/United States
- R01 CA109182-04/CA/NCI NIH HHS/United States
- R01 CA109182-03/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous