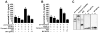A Shope Fibroma virus PYRIN-only protein modulates the host immune response - PubMed (original) (raw)
A Shope Fibroma virus PYRIN-only protein modulates the host immune response
Andrea Dorfleutner et al. Virus Genes. 2007 Dec.
Abstract
PYRIN domain (PYD) proteins have recently emerged as important signaling molecules involved in the development of innate immunity to intracellular pathogens through activation of inflammatory mediator pathways. ASC is the central adaptor protein, which links pathogen recognition by PYD-containing pathogen recognition receptors to the activation of downstream effectors, including activation of Caspase-1 and NF-kappaB. The cellular PYD-only protein 1 (cPOP1) can block the recruitment of ASC to activated PAN receptors and thereby functions as an endogenous inhibitor of the PYD-mediated signal transduction pathway. Here we describe the identification and characterization of a Shope Fibroma homolog to cPOP1. Like cPOP1, a Shope Fibroma virus-encoded POP (vPOP), co-localizes and directly associates with ASC and inhibits PYD-mediated signal transduction. Poxviruses are known to encode immune evasive proteins to promote host cell infection and suppression of the host immune response. Poxvirus-encoded vPOPs represent a novel class of immune evasive proteins and impair the host response by blocking Cryopyrin and ASC inflammasome-mediated activation of pro-Caspase-1 and subsequent processing of pro-interleukin (IL)-1beta, and expression of vPOPs causes activation of NF-kappaB.
Figures
Figure 1. The Shope Fibroma virus encodes a PYD-only protein
Comparison of viral and cellular PYD-only proteins. (A) Schematic representation of Myxoma virus (MV) POP (M013L), the Shope Fibroma virus (SFV) POP (gp013L), the Swinepox virus (SPV) POP (SPV14L), the Yaba-like disease virus (YLDV) POP (18L), the Mule deer poxvirus (DpV) POP (DPV83gp024), cPOP1, and ASC. (B) Clustal W alignment of M013L, gp013L, SPV14L, 18L, DPV83gp024, cPOP1 and the PYD of ASC. Black and grey boxes indicate identical and similar (conserved) amino-acid residues, respectively. The α-helices, as determined for the PYD of ASC are marked on top (44). (C) The presence of S013L-specific transcripts was determined in RK13 cells before and 16 hours post infection with the rabbit Fibroma virus (SFV) (MOI=10) by RT-PCR. RT-PCR was performed with S013L-specific primers and primers specific for β actin. RT: reverse transcriptase. A representative phase contrast image of uninfected RK13 cells (top) and RK13 cells infected with SFV (bottom) is shown. (D) Myc-tagged expression constructs for cPOP1, SFV gp013L and MV M013L were transiently transfected into HEK293T cells. 36 hours post transfection, normalized cell lysates were separated by SDS/PAGE and analyzed by immunoblot for expression of myc-tagged proteins.
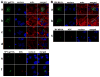
Figure 2. SFV gp013L co-localizes with ASC
Localization of epitope-tagged proteins was analyzed following transient transfection into RK13 cells. (A) Localization of SFV gp013L. Myc-tagged SFV gp013L was immunostained with a rabbit polyclonal anti-myc antibody and visualized with an Alexa Fluor 488-conjugated anti-rabbit antibody 48 hours post transfection (panel a) and 72 hours post transfection (panel b). Actin was visualized with Alexa Fluor 546-conjugated phalloidin and the nucleus was stained with ToPro-3. Shown is from left to right: Myc-tagged SFV gp013L (green), nucleus (blue), actin (red), and a merged image. 24 hours post transfection cells were also infected with SFV (MOI=10) and cells were immunostained as described above at 24 (panel c) and 48 hours (panel d) post infection (48 and 72 hours post transfection). Myc-tagged SFV gp013L and Flag-tagged ASC were immunostained 72 hours post transfection with rabbit polyclonal anti-myc and mouse monoclonal anti-Flag antibodies and visualized with Alexa Fluor 488 and 546-conjugated anti-rabbit and mouse antibodies, respectively. The nucleus was stained with ToPro-3. Shown is from left to right: Myc-tagged SFV gp013L (green), Flag-tagged ASC (red), nucleus (blue), and a merged image (panel e). 24 hours post transfection cells were also infected with SFV (MOI=10) and cells were immunostained as described above at 48 hours post infection (72 hours post transfection) (panel f). (B) Localization of MV M013L. Myc-tagged MV M013L was immunostained with a rabbit polyclonal anti-myc antibody and visualized with an Alexa Fluor 488-conjugated anti-rabbit antibody 48 hours post transfection (panel a) and 72 hours post transfection (panel b). Actin was visualized with Alexa Fluor 546-conjugated phalloidin and the nucleus was stained with ToPro-3. Shown is from left to right: Myc-tagged MV M013L (green), nucleus (blue), actin (red), and a merged image. Myc-tagged MV M013L and Flag-tagged ASC were immunostained 72 hours post transfection with rabbit polyclonal anti-myc and mouse monoclonal anti-Flag antibodies and visualized with Alexa Fluor 488 and 546-conjugated anti-rabbit and mouse antibodies, respectively. The nucleus was stained with ToPro-3. Shown is from left to right: Myc-tagged MV M013L (green), Flag-tagged ASC (red), nucleus (blue), and a merged image (panel c).
Figure 3. SFV gp013L associates with ASC
(A) In vivo binding between ASC and SFV gp013L. HEK293T cells were transiently transfected with HA-tagged ASC, myc-tagged SFV gp013L, and myc-tagged MV M013L, as indicated. 36 hours post transfection, clarified and normalized cell lysates were subjected to co-immunoprecipitation using immobilized anti-HA antibodies (Santa Cruz Biotechnology). Immune complexes were separated by SDS/PAGE transferred onto PVDF membranes and probed with anti-myc antibodies directly conjugated to HRP. Membranes were stripped and reprobed with anti-HA-HRP antibodies. 10% of the total lysate was run alongside the immunoprecipitation (IP). WB: western blot. (B) In vitro binding between ASC and SFV gp013L. SFV gp013L and MV M013L were in vitro translated, labeled with biotin, and subjected to in vitro GST-pull down assays using GST-ASC-CARD, GST-ASC-PYD, and GST immobilized to GSH sepharose, as indicated. Protein complexes were separated by SDS/PAGE, transferred onto PVDF membranes and bound proteins were visualized by immunoblotting with streptavidin-HRP and ECL-Plus (Amersham Pharmacia Biotech) detection. A membrane is stained with coomassie blue to visualize the GST fusion proteins. An asterisk denotes two degradation products, which is present in the ASC-PYD GST fusion protein purification, but which is not affecting this assay. A molecular weight standard is indicated to the right.
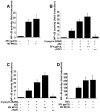
Figure 4. SFV gp013L inhibits Caspase-1-mediated processing of pro-IL-1β
HEK293N cells were transiently transfected in triplicates with expression constructs for pro-Caspase-1, murine pro-IL-1β, Cryopyrin (R260W), ASC, and SFV gp013L (A) or MV M013L (B), as indicated. 36 hours post transfection, secreted IL-1β was measured by ELISA (BD Pharmingen) from normalized culture supernatants using a standard curve generated with a recombinant IL-1β. Data are presented as picograms per milliliter of secreted IL-1β (mean SD; n=3). (C) HEK293N cells were transfected with the indicated expression constructs using the concentrations as used for the IL-1β assay (A, B), and cleared protein lysates were seperated by SDS/PAGE and immunoblotted with antibodies to detect expression of pro-caspase-1, Cryopyrin (R260W), ASC, SFV gp013L, and MV M013L. (+ and ++ indicate the two different expression levels of viral POPs).
Figure 5. SFV gp013L activates the transcription factor NF-κB
HEK293N cells were transiently transfected in 96-well plates in triplicates with the indicated expression constructs, including pNF-κB-LUC (Stratagene) and pRL-TK (Promega), keeping the total amount of DNA constant. (A) Cells were transfected with either a control plasmid or SFV gp013L or MV M013L expression plasmids, as indicated. (B) Cells were transfected with a control plasmid or ASC and Cryopyrin (R260W) in the presence or absence of increasing amounts of SFV gp013L, or cPOP1. (C) Cells were transfected with a control plasmid or ASC and Cryopyrin (R260W) in the presence or absence of increasing amounts of MV M013L, or cPOP1. (D) Cells were transfected with a control plasmid or SFV gp013L or MV M013L. Where indicated, 36 hours post transfection, cells were treated for 8 hours with 20 ng/ml recombinant human TNFα. Samples were analyzed using the Dual Glow Luciferase kit (Promega) in a Genios multimode plate reader (Tecan). Results are presented as fold induction of NF-κB relative to control transfected cells not induced with TNFα, normalized to tymidine kinase (TK) reporter gene activity. (mean SD; n=3).
Similar articles
- Cellular pyrin domain-only protein 2 is a candidate regulator of inflammasome activation.
Dorfleutner A, Bryan NB, Talbott SJ, Funya KN, Rellick SL, Reed JC, Shi X, Rojanasakul Y, Flynn DC, Stehlik C. Dorfleutner A, et al. Infect Immun. 2007 Mar;75(3):1484-92. doi: 10.1128/IAI.01315-06. Epub 2006 Dec 18. Infect Immun. 2007. PMID: 17178784 Free PMC article. - Co-regulation of NF-kappaB and inflammasome-mediated inflammatory responses by myxoma virus pyrin domain-containing protein M013.
Rahman MM, Mohamed MR, Kim M, Smallwood S, McFadden G. Rahman MM, et al. PLoS Pathog. 2009 Oct;5(10):e1000635. doi: 10.1371/journal.ppat.1000635. Epub 2009 Oct 23. PLoS Pathog. 2009. PMID: 19851467 Free PMC article. - Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.
de Lima JD, de Paula AGP, Yuasa BS, de Souza Smanioto CC, da Cruz Silva MC, Dos Santos PI, Prado KB, Winter Boldt AB, Braga TT. de Lima JD, et al. Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review. - Inhibiting the inflammasome: one domain at a time.
Dorfleutner A, Chu L, Stehlik C. Dorfleutner A, et al. Immunol Rev. 2015 May;265(1):205-16. doi: 10.1111/imr.12290. Immunol Rev. 2015. PMID: 25879295 Free PMC article. Review.
Cited by
- Evasion and interference: intracellular pathogens modulate caspase-dependent inflammatory responses.
Stewart MK, Cookson BT. Stewart MK, et al. Nat Rev Microbiol. 2016 Jun;14(6):346-59. doi: 10.1038/nrmicro.2016.50. Epub 2016 May 13. Nat Rev Microbiol. 2016. PMID: 27174147 Review. - The nucleic acid-sensing inflammasomes.
Xiao TS. Xiao TS. Immunol Rev. 2015 May;265(1):103-11. doi: 10.1111/imr.12281. Immunol Rev. 2015. PMID: 25879287 Free PMC article. Review. - Activation and Inhibition of the NLRP3 Inflammasome by RNA Viruses.
Choudhury SM, Ma X, Abdullah SW, Zheng H. Choudhury SM, et al. J Inflamm Res. 2021 Mar 26;14:1145-1163. doi: 10.2147/JIR.S295706. eCollection 2021. J Inflamm Res. 2021. PMID: 33814921 Free PMC article. Review. - The PYRIN Domain-only Protein POP1 Inhibits Inflammasome Assembly and Ameliorates Inflammatory Disease.
de Almeida L, Khare S, Misharin AV, Patel R, Ratsimandresy RA, Wallin MC, Perlman H, Greaves DR, Hoffman HM, Dorfleutner A, Stehlik C. de Almeida L, et al. Immunity. 2015 Aug 18;43(2):264-76. doi: 10.1016/j.immuni.2015.07.018. Epub 2015 Aug 11. Immunity. 2015. PMID: 26275995 Free PMC article. - The role of inflammasome modulation in virulence.
Lupfer CR, Kanneganti TD. Lupfer CR, et al. Virulence. 2012 May 1;3(3):262-70. doi: 10.4161/viru.20266. Epub 2012 May 1. Virulence. 2012. PMID: 22546900 Free PMC article. Review.
References
- Tschopp J, Martinon F, Burns K. Nat Rev Mol Cell Biol. 2003;4:95–104. - PubMed
- Ting JP, Davis BK. Annu Rev Immunol. 2004;23:387–414. - PubMed
- Inohara N, Nunez G. Nat Rev Immunol. 2003;3:371–381. - PubMed
- Conway KE, McConnell BB, Bowring CE, Donald CD, Warren ST, Vertino PM. Cancer Res. 2000;60:6236–6242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1R21-AI-067680/AI/NIAID NIH HHS/United States
- P20 RR016440/RR/NCRR NIH HHS/United States
- R03 AI067806/AI/NIAID NIH HHS/United States
- R 01-AI-56324/AI/NIAID NIH HHS/United States
- R01 AI056324/AI/NIAID NIH HHS/United States
- 1R03-AI-067806/AI/NIAID NIH HHS/United States
- R03 AI067806-02/AI/NIAID NIH HHS/United States
- 5P20-RR-016440/RR/NCRR NIH HHS/United States
- R21 AI067680-02/AI/NIAID NIH HHS/United States
- R21 AI067680/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous