A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth - PubMed (original) (raw)
A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth
Qianben Wang et al. Mol Cell. 2007.
Abstract
Androgen receptor (AR) is a ligand-dependent transcription factor that plays a key role in prostate cancer. Little is known about the nature of AR cis-regulatory sites in the human genome. We have mapped the AR binding regions on two chromosomes in human prostate cancer cells by combining chromatin immunoprecipitation (ChIP) with tiled oligonucleotide microarrays. We find that the majority of AR binding regions contain noncanonical AR-responsive elements (AREs). Importantly, we identify a noncanonical ARE as a cis-regulatory target of AR action in TMPRSS2, a gene fused to ETS transcription factors in the majority of prostate cancers. In addition, through the presence of enriched DNA-binding motifs, we find other transcription factors including GATA2 and Oct1 that cooperate in mediating the androgen response. These collaborating factors, together with AR, form a regulatory hierarchy that governs androgen-dependent gene expression and prostate cancer growth and offer potential new opportunities for therapeutic intervention.
Figures
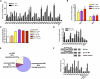
Figure 1. Map of AR Binding Sites on Human Chromosomes 21 and 22
RefSeq genes are shown in dark bars, and AR binding regions are shown as blue bars. Ten binding regions for further detailed analyses are indicated.
Figure 2. Characterization of AR Binding Sites on Chromosomes 21 and 22
(A) Standard ChIP assays of AR recruitment to various potential AR binding regions. LNCaP cells were treated with 100 nM DHT for 1 or 16 hr. ChIP assays were performed with anti-AR antibodies. Immunoprecipitated DNA was quantified by real-time PCR using primers (Table S1) spanning various potential AR binding regions. (B and C) Effects of DHT concentration on AR binding to chromatin and cell proliferation. (B) Androgen-depleted LNCaP cells were treated with 1, 10, or 100 nM DHT for 1 hr. ChIP assays were performed with an anti-AR antibody, and DNA precipitates were measured with real-time PCR using primers spanning the PSA enhancer and the B39 site. (C) LNCaP cells were cultured in hormone-depleted medium for 3 days and then treated with vehicle or DHT from 0.1 to 1000 nM for another 3 days. Cell proliferation was measured using the WST-1 assay. (D) Standard ChIP assays of Pol II recruitment to various potential AR binding regions. ChIP assays were performed with anti-Pol II antibodies. (E) Distribution of the types of AR binding motifs within AR binding sites and the chromosome 21 and 22 genomic background. (F) AR binding regions function as enhancers. Ten selected AR binding regions, mutant AR binding regions with deleted AREs (B21 and B39), and the FKBP5 enhancer were subcloned into the pGL3-promoter vector and transfected into androgen-depleted LNCaP cells. Empty pGL3 promoter vector was used as a negative control. Cells were stimulated with 100 nM DHT or vehicle for 24 hr. The data were presented as the mean ± SE of two to three replicates, (A)–(D) and (F).
Figure 3. Characterization of TMPRSS2-Regulatory Regions
(A) ChIP analyses of AR occupancy on the PSA and B39 in VCaP cells. VCaP cells (a prostate cancer cell line that expresses TMPRSS2:ERG fusion gene) were treated with 100 nM DHT for 1 hr. ChIP assays were performed with an anti-AR antibody. (B) Spatial communication between the TMPRSS2 enhancer and promoter. 5C was performed using fixed or EcoRI- or BtgI-digested chromatin from vehicle- or DHT-treated LNCaP cells. Primers (Table S1) flanking the –13.5 kb or 462 kb AR binding region and –700 bp promoter region were used to PCR amplify DNA after ligation. Control PCR was performed using chromatin before restriction enzyme digestion. (C) Schematic representation of the TMPRSS2 14 kb upstream regulatory region. Potential five ARE clusters and fourteen 1 kb fragments are shown. (D) ChIP analyses of AR recruitment to five potential ARE regions. ChIP assays were performed with anti-AR antibodies in LNCaP cells treated with vehicle or 100 nM DHT for 4 or 16 hr. (E) Systematic mapping of enhancer elements within the TMPRSS2 14 kb upstream regulatory region. Fourteen 1 kb TMPRSS2 upstream sequences were subcloned into the pGL3-promoter vector and transfected into LNCaP cells. Cells were treated with vehicle or 100 nM DHT for 24 hr. The data are presented as the mean ± SE of two to three replicates, (A), (D), and (E).
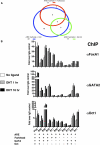
Figure 4. Collaborative Transcription Factors Are Recruited to AR Binding Regions
(A) Schematic representation of association of AR half-site and transcription factor motifs within AR binding regions. Each pairwise association is designated by a circle with different color. The area of the circle is proportional to the frequency of the association. (B) The recruitment of collaborative factors to AR binding regions. LNCaP cells were cultured in hormone-depleted medium for 3 days and then treated with vehicle or DHT for 1 and 16 hr. ChIP analyses were then performed with indicated antibodies or control IgG. DNA precipitates were measured by real-time PCR using primers spanning the PSA enhancer and nine AR binding regions on chromosomes 21 and 22. The results are shown as the average of two to three replicates ± SE. The occurrence of ARE, Forkhead, GATA, and Oct motifs in each region is also shown.
Figure 5. AR Interacts with Collaborative Transcription Factors In Vivo
(A) AR coimmunopreciptates with its collaborating factors in vivo. LNCaP cells were grown in the presence or absence of DHT for 24 hr. Whole-cell lysates were immunoprecipitated (IP) with indicated antibodies or control IgG. Western blot (WB) was performed with indicated antibodies. (B) AR-collaborating factor complexes form on chromatin. LNCaP cells were treated with or without DHT for 16 hr. ChIP assays were performed with anti-AR antibodies. The immunoprecipitated chromatin was eluted, reimmunoprecipitated with the indicated antibodies or control IgG, and amplified by PCR using primers flanking the PSA and B38 enhancer regions. (C and D) Colocalized _cis_-active elements are required for AR-dependent transcription. A wild-type PSA reporter construct and PSA reporter construct with deleted GATA or Oct motifs within the PSA enhancer or promoter regions (C) or B39 reporter wild-type construct and B39 reporters with GATA or Oct motif deletions (D) were transfected into LNCaP cells treated with vehicle or 100 nM DHT for 24 hr. Luciferase activities were determined, and results are presented as the mean ± SE of the triplicate experiments.
Figure 6. Functional Analyses of Collaborating Transcription Factors in Mediating AR-Dependent Transcription of the PSA and TMPRSS2 Genes
(A) Suppression of AR-collaborating factor levels by RNAi. LNCaP cells were transfected with siRNA targeting each factor and a control siRNA. Forty-eight hours posttransfection, cells were treated with or without 100 nM DHT for 16 hr, and western blots were performed using the antibodies indicated. (B) Effects of siRNA on PSA, TMPRSS2, and GADPH gene expression. Forty-eight hours after siRNA transfection, cells were treated with or without 1 or 100 nM DHT for 4 and 16 hr. Total RNA was isolated and amplified by real-time RT-PCR using transcript-specific primers (Table S1). The no-ligand control was measured at 4 hr. (C) Effects of silencing GATA2 and Oct1 on AR, Pol II, Oct1, and GATA2 recruitment to the PSA and TMPRSS2 enhancers. AR, Pol II, Oct1, and GATA2 ChIPs were performed after vehicle or 4 hr 100 nM DHT treatment of siLuc, siGATA2, or siOct1-transfected cells. Graphical representations of the mean ± SE of two to three independent experiments are shown in (B) and (C).
Figure 7. Functional Roles of Collaborating Transcription Factors in Mediating the PDE9A Gene Transcription and Prostate Cancer Cell Proliferation
(A) AR binding sites relative to the PDE9A gene. The black blocks represent AR binding sites. The PDE9A gene is shown in its 5′-3′ orientation, and the blue arrows indicate the direction of the gene (June 05, University of California, Santa Cruz [UCSC], known genes). (B) Effects of FoxA1, GATA2, and Oct1 silencing on PDE9A mRNA expression. siRNA-RTPCR analyses were performed as described in Figure 6B. (C) Effects of silencing GATA2 and Oct1 on AR, Pol II, Oct1, and GATA2 recruitment to the PDE9A enhancers. siRNA-ChIP analyses were performed as described in Figure 6C. (D) Effects of AR and cofactor silencing on androgen-stimulated cell cycle entry. Forty-eight hours after siRNA transfection, cells were treated with or without 1 or 10 nM DHT for 24 hr. Cells were then fixed, stained with propidium iodide, and analyzed by flow cytometry. Values represent the mean ± SE of two to three independent experiments (B) to (D).
Similar articles
- Identification of novel androgen response genes in prostate cancer cells by coupling chromatin immunoprecipitation and genomic microarray analysis.
Takayama K, Kaneshiro K, Tsutsumi S, Horie-Inoue K, Ikeda K, Urano T, Ijichi N, Ouchi Y, Shirahige K, Aburatani H, Inoue S. Takayama K, et al. Oncogene. 2007 Jun 28;26(30):4453-63. doi: 10.1038/sj.onc.1210229. Epub 2007 Feb 5. Oncogene. 2007. PMID: 17297473 - Amyloid precursor protein is a primary androgen target gene that promotes prostate cancer growth.
Takayama K, Tsutsumi S, Suzuki T, Horie-Inoue K, Ikeda K, Kaneshiro K, Fujimura T, Kumagai J, Urano T, Sakaki Y, Shirahige K, Sasano H, Takahashi S, Kitamura T, Ouchi Y, Aburatani H, Inoue S. Takayama K, et al. Cancer Res. 2009 Jan 1;69(1):137-42. doi: 10.1158/0008-5472.CAN-08-3633. Cancer Res. 2009. PMID: 19117996 - Integration of cap analysis of gene expression and chromatin immunoprecipitation analysis on array reveals genome-wide androgen receptor signaling in prostate cancer cells.
Takayama K, Tsutsumi S, Katayama S, Okayama T, Horie-Inoue K, Ikeda K, Urano T, Kawazu C, Hasegawa A, Ikeo K, Gojyobori T, Ouchi Y, Hayashizaki Y, Aburatani H, Inoue S. Takayama K, et al. Oncogene. 2011 Feb 3;30(5):619-30. doi: 10.1038/onc.2010.436. Epub 2010 Oct 4. Oncogene. 2011. PMID: 20890304 - Androgen receptor action in hormone-dependent and recurrent prostate cancer.
Agoulnik IU, Weigel NL. Agoulnik IU, et al. J Cell Biochem. 2006 Oct 1;99(2):362-72. doi: 10.1002/jcb.20811. J Cell Biochem. 2006. PMID: 16619264 Review. - Androgen receptor enhancer usage and the chromatin regulatory landscape in human prostate cancers.
Stelloo S, Bergman AM, Zwart W. Stelloo S, et al. Endocr Relat Cancer. 2019 May;26(5):R267-R285. doi: 10.1530/ERC-19-0032. Endocr Relat Cancer. 2019. PMID: 30865928 Review.
Cited by
- Increased Hospitalization and Mortality from COVID-19 in Prostate Cancer Patients.
Chakravarty D, Ratnani P, Sobotka S, Lundon D, Wiklund P, Nair SS, Tewari AK. Chakravarty D, et al. Cancers (Basel). 2021 Apr 1;13(7):1630. doi: 10.3390/cancers13071630. Cancers (Basel). 2021. PMID: 33915795 Free PMC article. - Interrogating genomic and epigenomic data to understand prostate cancer.
Kim J, Yu J. Kim J, et al. Biochim Biophys Acta. 2012 Apr;1825(2):186-96. doi: 10.1016/j.bbcan.2011.12.003. Epub 2012 Jan 3. Biochim Biophys Acta. 2012. PMID: 22240201 Free PMC article. Review. - Targeting pioneering factor and hormone receptor cooperative pathways to suppress tumor progression.
Shah S, Prasad S, Knudsen KE. Shah S, et al. Cancer Res. 2012 Mar 1;72(5):1248-59. doi: 10.1158/0008-5472.CAN-11-0943. Epub 2012 Jan 18. Cancer Res. 2012. PMID: 22258452 Free PMC article. - Sex differences in SARS-CoV-2 infection rates and the potential link to prostate cancer.
Chakravarty D, Nair SS, Hammouda N, Ratnani P, Gharib Y, Wagaskar V, Mohamed N, Lundon D, Dovey Z, Kyprianou N, Tewari AK. Chakravarty D, et al. Commun Biol. 2020 Jul 8;3(1):374. doi: 10.1038/s42003-020-1088-9. Commun Biol. 2020. PMID: 32641750 Free PMC article. Review. - Modeling cis-regulation with a compendium of genome-wide histone H3K27ac profiles.
Wang S, Zang C, Xiao T, Fan J, Mei S, Qin Q, Wu Q, Li X, Xu K, He HH, Brown M, Meyer CA, Liu XS. Wang S, et al. Genome Res. 2016 Oct;26(10):1417-1429. doi: 10.1101/gr.201574.115. Epub 2016 Jul 27. Genome Res. 2016. PMID: 27466232 Free PMC article.
References
- Balk SP, Ko YJ, Bubley GJ. Biology of prostate-specific antigen. J. Clin. Oncol. 2003;21:383–391. - PubMed
- Boyes J, Byfield P, Nakatani Y, Ogryzko V. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature. 1998;396:594–598. - PubMed
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122:33–43. - PubMed
- Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, et al. Genome-wide analysis of estrogen receptor binding sites. Nat. Genet. 2006;38:1289–1297. - PubMed
- Cirillo LA, Lin FR, Cuesta I, Friedman D, Jarnik M, Zaret KS. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol. Cell. 2002;9:279–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 CA090381/CA/NCI NIH HHS/United States
- P50 CA 69568/CA/NCI NIH HHS/United States
- P50 CA 90381/CA/NCI NIH HHS/United States
- R01 HG004069-01/HG/NHGRI NIH HHS/United States
- R01 HG004069-02/HG/NHGRI NIH HHS/United States
- R01 HG004069/HG/NHGRI NIH HHS/United States
- P50 CA069568/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials