DNA damage-dependent acetylation and ubiquitination of H2AX enhances chromatin dynamics - PubMed (original) (raw)
. 2007 Oct;27(20):7028-40.
doi: 10.1128/MCB.00579-07. Epub 2007 Aug 20.
Satoshi Tashiro, Akemi Kakino, Hiroki Shima, Naduparambil Jacob, Ravindra Amunugama, Kristine Yoder, Shunsuke Izumi, Isao Kuraoka, Kiyoji Tanaka, Hiroshi Kimura, Masae Ikura, Shuichi Nishikubo, Takashi Ito, Akihiko Muto, Kiyoshi Miyagawa, Shunichi Takeda, Richard Fishel, Kazuhiko Igarashi, Kenji Kamiya
Affiliations
- PMID: 17709392
- PMCID: PMC2168918
- DOI: 10.1128/MCB.00579-07
DNA damage-dependent acetylation and ubiquitination of H2AX enhances chromatin dynamics
Tsuyoshi Ikura et al. Mol Cell Biol. 2007 Oct.
Abstract
Chromatin reorganization plays an important role in DNA repair, apoptosis, and cell cycle checkpoints. Among proteins involved in chromatin reorganization, TIP60 histone acetyltransferase has been shown to play a role in DNA repair and apoptosis. However, how TIP60 regulates chromatin reorganization in the response of human cells to DNA damage is largely unknown. Here, we show that ionizing irradiation induces TIP60 acetylation of histone H2AX, a variant form of H2A known to be phosphorylated following DNA damage. Furthermore, TIP60 regulates the ubiquitination of H2AX via the ubiquitin-conjugating enzyme UBC13, which is induced by DNA damage. This ubiquitination of H2AX requires its prior acetylation. We also demonstrate that acetylation-dependent ubiquitination by the TIP60-UBC13 complex leads to the release of H2AX from damaged chromatin. We conclude that the sequential acetylation and ubiquitination of H2AX by TIP60-UBC13 promote enhanced histone dynamics, which in turn stimulate a DNA damage response.
Figures
FIG. 1.
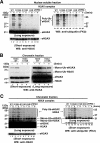
TIP60 regulates the acetylation of H2AX(K5) upon DNA damage. (A) The mock control, eH2AX, and eH2A complexes were immunoaffinity purified from the nuclear soluble fraction of HeLa cells treated without (−) or with (+) IR at 12 Gy followed by a 5-min recovery. Immunoblotting analysis was performed with anti-TIP60 (top) and anti-FLAG (bottom) antibodies. (B) The eH2AX complex immunoaffinity purified from the nuclear soluble fraction of HeLa cells treated with IR (12 Gy) followed by a 5-min recovery. Proteins were analyzed by immunoblotting using anti-acetyl-K5 of H2A antibody (top). Unirradiated cells (−) were used as controls. eH2AX was used as a loading control (bottom). (C) Depletion of TIP60 by TIP60 siRNA. Shown are anti-TIP60 immunoblots of lysates from TIP60 siRNA (siTIP60.1)-expressing and mock control cells prepared at the indicated times after IR at 12 Gy (top). GAPDH was used as a loading control (bottom). (D) Effect of the depletion of TIP60 on the acetylation of H2AX after IR. TIP60-specific siRNA (siTIP60.1)-expressing and mock control cells transfected with a FLAG-H2AX expression vector were treated with IR at 12 Gy and allowed to recover for 5 min. Immunoprecipitation was carried out using the anti-FLAG antibody, and proteins were analyzed by immunoblotting with antibodies against H2A acetylated on K5 (top) or total H2AX (bottom). Cells were treated with sodium butyrate (final concentration, 5 mM) for the detection of acetylation. (E) Western blot analysis of the TIP60 complex by use of anti-H2AX (top) and anti-HA (bottom; loading control) antibodies. Shown are the mock control and the TIP60 complex immunoaffinity purified from nuclear extract of HeLa cells stably expressing FLAG-HA eTIP60 with IR at 12 Gy followed by a 5-min recovery. Bands that reacted with anti-H2AX antibody are indicated by an asterisk and a bar. eTIP60 was used as a loading control (bottom). WB, Western blot.
FIG. 2.
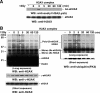
Acetylation of H2AX regulated by TIP60 is required for the ubiquitination of H2AX upon DNA damage. (A and C) Wild-type eH2AX (lanes 1, 2, 11, and 12) and mutants eH2AX(S139A) (lanes 3, 4, 13, and 14), eH2AX(K119R) (lanes 5, 6, 15, and 16), and eH2AX(K5R) (lanes 7, 8, 17, and 18) were immunoaffinity purified from the nuclear soluble (A) and chromatin (C) fractions of cells stably expressing wild-type or mutant forms of eH2AX treated with IR (12 Gy) followed by a 5-min recovery. Wild-type eH2AX was also purified from TIP60 knockdown cells (siTIP60.1) expressing wild-type eH2AX (lanes 9, 10, 19, and 20). Proteins were analyzed by immunoblotting using anti-H2AX (left) and anti-ubiquitin (FK2) (right) antibodies. Asterisks indicate that the signals reacted with both anti-H2AX and antiubiquitin (FK2) antibodies. eH2AX was used as a loading control (bottom). (A) Unmodified eH2AX and polyubiquitinated eH2AX (poly-Ub-eH2AX) are indicated. (B) The monoubiquitination status of immunoaffinity-purified wild-type eH2AX (lanes 1, 2, 5, and 6) and the K119R mutant (lanes 3, 4, 7, and 8) from the chromatin fractions of cells treated with IR (12 Gy) followed by a 5-min recovery was analyzed by immunoblotting using anti-H2AX antibody. Unmodified eH2AX and mono-Ub-eH2AX were detected together with unmodified H2AX and mono-Ub-H2AX. (C) Poly-Ub-eH2AX and endogenous H2AX are indicated. WT, wild type; WB, Western blot.
FIG. 3.
H2AX is acetylated independent of phosphorylation and ubiquitination upon DNA damage. Wild-type and mutant eH2AX proteins (S139A, K119R, and K5R) were immunoaffinity purified from the chromatin fraction of HeLa cells treated with IR at 12 Gy followed by a 5-min recovery, and the acetylation status was analyzed by immunoblotting with the anti-acetyl-K5 of H2A (top). eH2AX was used as a loading control (bottom). Cells were treated with sodium butyrate (final concentration, 5 mM) for the detection of acetylation. WT, wild type; WB, Western blot.
FIG. 4.
Time course of acetylation, ubiquitination, and phosphorylation of H2AX after induction of DSBs. Wild-type eH2AX was immunoaffinity purified from the chromatin-bound fraction derived from HeLa cells stably expressing eH2AX at the indicated times of recovery after IR (12 Gy). (A) The acetylation status was analyzed by immunoblotting with the anti-acetyl-K5 of H2A (top). eH2AX was used as a loading control (bottom). Cells were treated with sodium butyrate (final concentration, 5 mM) for the detection of acetylation. (B) Proteins were analyzed by immunoblotting using anti-H2AX (top and bottom in left gel), anti-γ-H2AX (middle in left gel), and antiubiquitin (FK2) (right) antibodies. eH2AX was used as a loading control (bottom in left gel). Asterisks indicate the bands reacted with both anti-H2AX and antiubiquitin (FK2) antibodies. WB, Western blot.
FIG. 5.
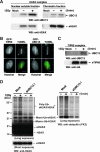
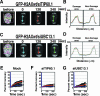
UBC13, a ubiquitin-conjugating enzyme, interacts with TIP60 in damaged chromatin. (A) Affinity purification of eH2AX from the nuclear soluble (left) and chromatin (right) fractions of HeLa cells treated with IR at 12 Gy followed by a 5-min recovery. As a control, a mock purification was performed using nontransfected HeLa cells. UBC13 was detected by immunoblotting using anti-UBC13 antibody (top). eH2AX was used as a loading control (bottom). (B) Accumulation of GFP-TIP60 (left) or GFP-UBC13 (right) in GM02063 cells at sites containing DSBs after a 5-min recovery from laser UVA microirradiation. TUNEL staining was performed to detect DSBs induced by microirradiation. GFP-TIP60/UBC13 and TUNEL signals are shown in green and red, respectively, in merged images. Scale bars, 10 μm. (C) DSBs facilitate the interaction of UBC13 with the TIP60 complex. Immunoblot analysis of the mock control and the TIP60 complex affinity purified from the nuclear soluble fraction of cells treated with IR (12 Gy) followed by a 5-min recovery using anti-UBC13 antibody (top). eTIP60 was used as a loading control (bottom). (D) Wild-type eH2AX was affinity purified from the chromatin fraction of UBC13-specific siRNA (siUBC13.1)-expressing cells or the mock control cells treated with IR (12 Gy) followed by a 5-min recovery. Proteins were analyzed by immunoblotting using anti-H2AX (left) and antiubiquitin (FK2) (right) antibodies. WB, Western blot.
FIG. 6.
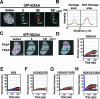
In vivo dynamics of H2AX regulated by ubiquitination and acetylation of H2AX after induction of DSBs by microirradiation. (A) iFRAP analysis of GFP-H2AX in combination with microirradiation. Confocal images taken at indicated times after microirradiation are shown. DSBs were induced in GM02063 cells expressing GFP-H2AX by laser UVA microirradiation in the area indicated by a yellow box. Immediately after the induction of DSBs, fluorescence was bleached in the areas indicated by red boxes with the 488-nm laser line of an Ar laser. Scale bar, 5 μm. (B) Graph representation of fluorescence intensity versus distance along the arrows depicted in panel A. (C) FRAP analysis of GFP-H2AX after laser UVA microirradiation. GFP-H2AX was imaged before microirradiation, immediately after bleaching, and 120 s after bleaching (see the video in the supplemental material). Scale bar, 5 μm. (D) Quantitative analysis of fluorescence recovery curves after bleaching of irradiated (1, red line) and control (2, blue line) areas. Values represent averages ± standard errors for 14 cells. (E to H) Fluorescence recovery curves for GFP-H2A.1 (n = 11) (E), GFP-H2AX(K5R) (n = 12) (F), GFP-H2AX(K119R) (n = 10) (G), and GFP-H2AX(S139A) (n = 15) (H) in GM02063 cells over time in the microirradiated (red lines) and control (2, blue lines) regions.
FIG. 7.
TIP60 and UBC13 are required for the release of H2AX upon DNA damage. (A to D) iFRAP analysis of GFP-H2AX after microirradiation in GM02063 cells expressing TIP60 (siTIP60.1)- or UBC13 (siUBC13.1)-specific siRNAs was performed as described for Fig. 6A and B. Depletion of TIP60 or UBC13 suppressed the DSB-induced release of GFP-H2AX. Scale bars, 5 μm. (E to G) Fluorescence recovery curves for GFP-H2AX in the microirradiated (red lines) and control (blue lines) regions in mock-transfected cells (n = 15) (E) and for GFP-H2AX in GM02063 cells expressing TIP60-specific siRNA (siTIP60.1) (n = 10) (F) or UBC13-specific siRNA (siUBC13.1) (n = 12) (G).
Similar articles
- Acetylation of Histone H2AX at Lys 5 by the TIP60 Histone Acetyltransferase Complex Is Essential for the Dynamic Binding of NBS1 to Damaged Chromatin.
Ikura M, Furuya K, Matsuda S, Matsuda R, Shima H, Adachi J, Matsuda T, Shiraki T, Ikura T. Ikura M, et al. Mol Cell Biol. 2015 Dec;35(24):4147-57. doi: 10.1128/MCB.00757-15. Epub 2015 Oct 5. Mol Cell Biol. 2015. PMID: 26438602 Free PMC article. - Coordinated Regulation of TIP60 and Poly(ADP-Ribose) Polymerase 1 in Damaged-Chromatin Dynamics.
Ikura M, Furuya K, Fukuto A, Matsuda R, Adachi J, Matsuda T, Kakizuka A, Ikura T. Ikura M, et al. Mol Cell Biol. 2016 May 2;36(10):1595-607. doi: 10.1128/MCB.01085-15. Print 2016 May 15. Mol Cell Biol. 2016. PMID: 26976643 Free PMC article. - Sirt1 physically interacts with Tip60 and negatively regulates Tip60-mediated acetylation of H2AX.
Yamagata K, Kitabayashi I. Yamagata K, et al. Biochem Biophys Res Commun. 2009 Dec 25;390(4):1355-60. doi: 10.1016/j.bbrc.2009.10.156. Epub 2009 Nov 4. Biochem Biophys Res Commun. 2009. PMID: 19895790 - Crosstalk between histone modifications during the DNA damage response.
van Attikum H, Gasser SM. van Attikum H, et al. Trends Cell Biol. 2009 May;19(5):207-17. doi: 10.1016/j.tcb.2009.03.001. Epub 2009 Apr 1. Trends Cell Biol. 2009. PMID: 19342239 Review. - Role of histone acetyltransferases MOF and Tip60 in genome stability.
Mir US, Bhat A, Mushtaq A, Pandita S, Altaf M, Pandita TK. Mir US, et al. DNA Repair (Amst). 2021 Nov;107:103205. doi: 10.1016/j.dnarep.2021.103205. Epub 2021 Aug 8. DNA Repair (Amst). 2021. PMID: 34399315 Review.
Cited by
- Coilin levels modulate cell cycle progression and γH2AX levels in etoposide treated U2OS cells.
Velma V, Carrero ZI, Allen CB, Hebert MD. Velma V, et al. FEBS Lett. 2012 Sep 21;586(19):3404-9. doi: 10.1016/j.febslet.2012.07.054. Epub 2012 Aug 7. FEBS Lett. 2012. PMID: 22986342 Free PMC article. - Substrate recognition and function of the R2TP complex in response to cellular stress.
von Morgen P, Hořejší Z, Macurek L. von Morgen P, et al. Front Genet. 2015 Feb 25;6:69. doi: 10.3389/fgene.2015.00069. eCollection 2015. Front Genet. 2015. PMID: 25767478 Free PMC article. Review. - The stress-responsive gene ATF3 regulates the histone acetyltransferase Tip60.
Cui H, Guo M, Xu D, Ding ZC, Zhou G, Ding HF, Zhang J, Tang Y, Yan C. Cui H, et al. Nat Commun. 2015 Apr 13;6:6752. doi: 10.1038/ncomms7752. Nat Commun. 2015. PMID: 25865756 Free PMC article. - Effective elimination of cancer stem cells by a novel drug combination strategy.
Yuan S, Wang F, Chen G, Zhang H, Feng L, Wang L, Colman H, Keating MJ, Li X, Xu RH, Wang J, Huang P. Yuan S, et al. Stem Cells. 2013 Jan;31(1):23-34. doi: 10.1002/stem.1273. Stem Cells. 2013. PMID: 23132831 Free PMC article. - The chromatin-binding protein PHF6 functions as an E3 ubiquitin ligase of H2BK120 via H2BK12Ac recognition for activation of trophectodermal genes.
Oh S, Boo K, Kim J, Baek SA, Jeon Y, You J, Lee H, Choi HJ, Park D, Lee JM, Baek SH. Oh S, et al. Nucleic Acids Res. 2020 Sep 18;48(16):9037-9052. doi: 10.1093/nar/gkaa626. Nucleic Acids Res. 2020. PMID: 32735658 Free PMC article.
References
- Ahmad, K., and S. Henikoff. 2002. The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol. Cell 9:1191-1200. - PubMed
- Angelov, D., A. Molla, P. Y. Perche, F. Hans, J. Cote, S. Khochbin, P. Bouvet, and S. Dimitrov. 2003. The histone variant macroH2A interferes with transcription factor binding and SWI/SNF nucleosome remodeling. Mol. Cell 11:1033-1041. - PubMed
- Aten, J. A., J. Stap, P. M. Krawczyk, C. H. van Oven, R. A. Hoebe, J. Essers, and R. Kanaar. 2004. Dynamics of DNA double-strand breaks revealed by clustering of damaged chromosome domains. Science 303:92-95. - PubMed
- Bassing, C. H., and F. W. Alt. 2004. H2AX may function as an anchor to hold broken chromosomal DNA ends in close proximity. Cell Cycle 3:149-153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous