Severe acute respiratory syndrome coronavirus evades antiviral signaling: role of nsp1 and rational design of an attenuated strain - PubMed (original) (raw)
Severe acute respiratory syndrome coronavirus evades antiviral signaling: role of nsp1 and rational design of an attenuated strain
Marc G Wathelet et al. J Virol. 2007 Nov.
Abstract
The severe acute respiratory syndrome (SARS) epidemic was caused by the spread of a previously unrecognized infectious agent, the SARS-associated coronavirus (SARS-CoV). Here we show that SARS-CoV could inhibit both virus- and interferon (IFN)-dependent signaling, two key steps of the antiviral response. We mapped a strong inhibitory activity to SARS-CoV nonstructural protein 1 (nsp1) and show that expression of nsp1 significantly inhibited the activation of all three virus-dependent signaling pathways. We show that expression of nsp1 significantly inhibited IFN-dependent signaling by decreasing the phosphorylation levels of STAT1 while having little effect on those of STAT2, JAK1, and TYK2. We engineered an attenuated mutant of nsp1 in SARS-CoV through reverse genetics, and the resulting mutant virus was viable and replicated as efficiently as wild-type virus in cells with a defective IFN response. However, mutant virus replication was strongly attenuated in cells with an intact IFN response. Thus, nsp1 is likely a virulence factor that contributes to pathogenicity by favoring SARS-CoV replication.
Figures
FIG. 1.
(A) VeroE6 cells, uninfected (U) or infected for 1 h with WT SARS-CoV (MOI, 3) or SeV, were further incubated at 37°C. Protein extracts were harvested at the indicated times and analyzed by immunoblotting. Over the time course, the 15-kDa and 56-kDa proteins were more induced by SeV than by SARS-CoV (7.4- and 6.2-fold, respectively) (equal amounts of protein were verified by c-Jun and IκB levels [see panel C]). (B) RNAs from cells treated as described for panel A were analyzed by Northern blotting with the indicated probes. The signals for ISG54 and ISG15 mRNAs in SARS-CoV-infected samples are not bands but a smear that corresponds to low-level degradation of the much more intense rRNA signals that happen to also be detected by the ISG54 probe (28S rRNA) and the ISG15 probe (28S and 18S rRNAs). RNA sizes of the ISG bands are indicated on the left, in kb. Dashes mark the positions of the nine SARS-CoV RNAs. (C) Immunoblots as described for panel A were probed with anti-(phospho)-c-Jun or anti-(phospho)-IκB antibodies. (D) Structure of SARS-CoV replicase (see the text for more details). (E) Immunoblot analysis of extracts from 293T cells transfected with empty plasmid (−) or plasmids for the indicated Flag-nsp constructs. The predicted sizes of the nsps are indicated below the gel, in kDa. Different extract dilutions were used as follows: nsp1, 10×; nsp2, 150×; nsp3, 50×; nsp4, 100×; nsp5, 60×; nsp6, 10×; nsp7, 4×; nsp8, 4×; nsp9, 5×; nsp10, 30×; nsp11, 2×; nsp12, 2×; nsp13, 2×; nsp14, 200×; nsp15, 200×; and nsp16, 2×. (F) 293T cells transfected with empty plasmid (−) or plasmids for the indicated Flag-nsp constructs, together with −110IFNβCAT, were left uninfected (U) or infected with SeV for 18 h, and reporter activity was determined after normalization for transfection efficiency, not nsp expression levels.
FIG. 2.
293T cells were transfected with empty vector (Vec.) or a vector for Flag-nsp1 expression (nsp1), together with different reporters and the CMV-lacZ and pcDβA-luciferase transfection efficiency controls (used in Fig. 1, 3, 4, and 8). (A) β-Galactosidase activity was assayed in a 150-μl reaction mix with _ortho_-nitrophenyl-β-galactopyranoside in a 96-well plate and read on an enzyme-linked immunosorbent assay reader at 405 nm every 5 min after the start of the reaction. Values in the linear range of the assay were normalized to the average (0.634) for cells transfected with empty vector, and nsp1 expression resulted in an average decrease to a value of 0.060, with a standard deviation of 0.040. (B) Luciferase activity was assayed in a 60-μl reaction mix by using a luciferase assay kit (Roche) and read in a Femtomaster FB12 instrument (Zylux). Values in the linear range of the assay were normalized to the average (5,899,000) for cells transfected with empty vector, and nsp1 expression resulted in an 11% increase, which was not statistically significant, to a value of 6,562,000 (standard deviation of 2,482,000). (C) 293T cells were transfected with empty vector (Vec.) or a vector for Flag-nsp1 expression (nsp1), together with the G5E1bCAT reporter, which contains five copies of the upstream activating sequence, a 17-mer binding site for Gal4, and a construct expressing the DNA-binding domain of Gal4 (amino acids 1 to 147) fused to the full-length coding region of the 65-kDa subunit of NF-κB (Gal4p65). CAT activity was assayed and normalized to luciferase activity. The average activity of Gal4p65 activating the G5E1bCAT reporter was increased 28% when nsp1 was coexpressed, but the increase was not statistically significant (P = 0.28).
FIG. 3.
(A) 293T cells transfected with empty vector (Vec.) or vector for nsp1 or nsp3, together with PRDIIx3CAT, were left uninfected (U), infected with SeV, or treated with 10 ng/ml of TNF for 18 h, and reporter activity was determined. (B) 293T cells transfected with empty vector (Vec.) or vector for nsp1 or nsp3, together with P31x3CAT, were left uninfected (U) or infected with SeV for 18 h, and reporter activity was determined. (C) 293T cells transfected with empty vector (Vec.) or vectors for expression of Flag-nsp1, Flag-nsp3, and/or H6IRF3 were left uninfected (−) or infected with SeV (+) for 6 h, and extracts were analyzed by deoxycholate-PAGE and immunoblotting. (D) 293T cells transfected with Gal4-IRF7B, G5E1bCAT, and empty vector (Vec.) or a vector for nsp1 were left uninfected (U) or infected with SeV for 18 h, and reporter activity was determined. Extracts were also analyzed by immunoblotting with an anti-IRF7 antibody, which detects both Gal4-IRF7 and endogenous IRF7 (bottom). (E) 293T cells transfected with empty vector (Vec.) or vectors for nsp1, nsp3, and/or c-Jun were left uninfected (−) or infected with SeV (+) for 6 h, and expression and phosphorylation of c-Jun were determined by immunoblotting. GAPDH expression did not vary under these conditions. (F) VeroE6 cell extracts used for Fig. 1A and extracts of 293T cells transfected with empty vector (Vec.) or Flag-nsp1 were analyzed by immunoblotting with anti-nsp1 (VU231) (30) and anti-STAT1 antibodies. (G) VeroE6 cell extracts used for Fig. 1A and extracts of 293T cells transfected with empty vector (Vec.) or Flag-nsp3 were analyzed by immunoblotting with two anti-nsp3 antibodies (VU235 [top] and VU233 [middle], specific for the C and N termini, respectively [30]) and anti-STAT1 antibody [bottom].
FIG. 4.
(A) VeroE6 cells were left uninfected or were infected for 1 h with WT SARS-CoV (MOI, ∼5) and further incubated for 8 h. IFN-α (2,000 U/ml) was then added for 2, 4.5, and 7 h before immunoblot analysis. (B) 293T cells transfected with 9-27 ISREx4CAT and empty vector (−) or Flag-nsp constructs were left untreated (Co) or treated with 500 U/ml of IFN-α for 18 h, and reporter activity was determined. (C) 293T cells transfected with ISG15 ISREx3CAT and empty vector (Vec.) or vector for nsp1 were left untreated (Co), infected with SeV, or treated with 500 U/ml of IFN-α or IFN-γ for 18 h, and reporter activity was determined.
FIG. 5.
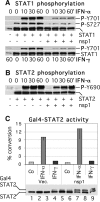
(A) 293T cells transfected with empty vector (−) or vectors for expression of nsp1 and/or STAT1α were treated with 2,000 U/ml of IFN-α or IFN-γ for 0, 10, 30, and 60 min, and extracts were analyzed by immunoblotting. (B) 293T cells transfected with empty vector (−) or vectors for nsp1 and/or STAT2 were treated with 2,000 U/ml of IFN-α for 0, 10, 30, and 60 min, and extracts were analyzed by immunoblotting. (C) 293T cells transfected with Gal4-STAT2, G5E1bCAT, and empty vector (Vec.) or a vector for nsp1 were left untreated (Co) or treated with 500 U/ml of IFN-α or IFN-γ for 18 h, and reporter activity was determined. Extracts were also analyzed by immunoblotting with an anti-STAT2 antibody, which detects the Gal4-STAT2 fusion and endogenous STAT2. Lanes 1 to 3, cells transfected with empty vector; lanes 4 to 6, cells transfected with Gal4-STAT2; lanes 7 to 9, cells transfected with Gal4-STAT2 and nsp1. Cells were left untreated (lanes 1, 4, and 7) or were treated for 18 h with 500 U/ml of IFN-α (lanes 2, 5, and 8) or IFN-γ (lanes 3, 6, and 9).
FIG. 6.
(A) 293T cells were transfected with the indicated amounts of a JAK1 expression vector and with empty vector (−) or a vector for nsp1 (+), and the levels of JAK1 were determined by immunoblotting. (B) 293T cells transfected with empty vector (−) or vector for JAK1 or TYK2, with or without nsp1, were treated with 2,000 U/ml of IFN-α for 0, 10, 30, and 60 min, and extracts were analyzed by immunoblotting. To compensate for the effect of nsp1 on JAK1 levels, 10 μl of extracts transfected with 7 μg of JAK1 and 30 μl of extracts cotransfected with 15 μg of JAK1 and 15 μg of nsp1 were loaded in the gel.
FIG. 7.
293T cells transfected with empty vector (Vec.) or a vector for nsp1, together with pcDβA-eGFP, for 48 h were fixed, stained with PI in the presence of RNase A, and analyzed using flow cytometry to determine DNA content and the distribution of the cell population in the various phases of the cell cycle.
FIG. 8.
(A) 293T cells were transfected with empty vector (Vec.) or vectors for WT or mutant Flag-nsp1 and reporters, as indicated. Cells transfected with −110IFNβCAT were left uninfected (U) or infected with SeV, and cells transfected with 9-27 ISREx4CAT were left untreated (Co) or treated with 500 U/ml of IFN-α for 18 h before reporter activity was determined. These extracts were also analyzed for nsp1 levels by immunoblotting (bottom). (B) Ninety percent confluent VeroE6 cells were infected with the indicated viruses at an MOI of ∼0.5. Supernatants were harvested at the indicated times postinfection, and their titers were determined by plaque assay. (C) VeroE6 cells were left uninfected (U) or were infected at an MOI of ∼3, and extracts were harvested at the indicated times for immunoblot analysis.
FIG. 9.
(A) Immunoblot analysis of extracts from confluent VeroE6 or Calu-3 cells that were left uninfected (U) or infected with WT or m1p1 SARS-CoV (MOI, ∼1) for the indicated times (h) or with SeV for 12 h [or 6 h in the case of anti-(phospho)-STAT1]. (B) Immunoblot analysis of extracts from confluent VeroE6 or Calu-3 cells that were left uninfected (U) or infected with WT or m1p1 SARS-CoV at the indicated MOIs (∼0.01, 0.1, or 1) for 24 h or with SeV for 18 h [or 12 h in the case of anti-(phospho)-STAT1]. (C) Confluent VeroE6 or Calu-3 cells were left uninfected (U) or infected with WT or m1p1 SARS-CoV (MOI, ∼0.1 for VeroE6 cells and ∼1 for Calu-3 cells) for 24 h and treated with 500 U/ml IFN-α from 16 to 24 hpi (the last 8 h). Cell extracts were analyzed by immunoblotting. (D) Immunoblot analysis of extracts from the VeroE6 and Calu-3 cells used for panel C that were run side by side to directly compare the relative levels of expression of ISG15 is uninfected cells (U), cells infected with SARS-CoV ml (ml), and cells treated with IFN. (E) Immunoblot analysis of ISG15 ectopically expressed in 293T cells by transfection of a construct driven by the CMV promoter, pcDβA-hISG15, in the presence or absence of constructs for expression of nspl or ml, as indicated.
FIG. 10.
(A and B) Confluent VeroE6 and Calu-3 cells were left untreated (lanes 0 and 1) or pretreated with increasing concentrations of IFN-α (lanes 2 to 9, 3, 10, 30, 100, 300, 1,000, 3,000, and 10,000 U/ml) for 24 h. Cells were left uninfected (U) or were infected with VSV for 1 h (MOI, ∼1), after which all cells were washed and further incubated for 17 h, when supernatants were titrated by limiting dilution (A) and cell extracts were analyzed by immunoblotting (B). (C to E) Confluent VeroE6 and Calu-3 cells were pretreated with increasing concentrations of IFN-α as described above, infected with WT SARS-CoV (W) or m1 (m) for 1 h (MOI, ∼1) or left uninfected (U), washed, and further incubated for 17 h, when supernatants were titrated by limiting dilution (C and D) and cell extracts were analyzed by immunoblotting (E). (F) Confluent VeroE6 and Calu-3 cells were infected with WT SARS-CoV and m1 (MOI, ∼1) for 12 h, and titers were determined by limiting dilution. (G) Confluent VeroE6 and Calu-3 cells were infected with WT SARS-CoV and m1 at a low MOI of ∼4 × 10−5, and supernatants were harvested at the indicated times (hpi) and titrated by limiting dilution. Each data point represents the average titer of 9 samples for VeroE6 cells with titers of at least 7 × 105 PFU/ml at 48 hpi or the average titer of 12 samples for Calu-3 cells (for Calu-3 cells, two WT SARS-CoV samples and six SARS-CoV m1 samples had undetectable levels of virus, i.e., <4.4 PFU/ml).
Similar articles
- Severe acute respiratory syndrome coronavirus nsp1 suppresses host gene expression, including that of type I interferon, in infected cells.
Narayanan K, Huang C, Lokugamage K, Kamitani W, Ikegami T, Tseng CT, Makino S. Narayanan K, et al. J Virol. 2008 May;82(9):4471-9. doi: 10.1128/JVI.02472-07. Epub 2008 Feb 27. J Virol. 2008. PMID: 18305050 Free PMC article. - SARS-CoV-2 Nonstructural Protein 1 Inhibits the Interferon Response by Causing Depletion of Key Host Signaling Factors.
Kumar A, Ishida R, Strilets T, Cole J, Lopez-Orozco J, Fayad N, Felix-Lopez A, Elaish M, Evseev D, Magor KE, Mahal LK, Nagata LP, Evans DH, Hobman TC. Kumar A, et al. J Virol. 2021 Jun 10;95(13):e0026621. doi: 10.1128/JVI.00266-21. Epub 2021 Jun 10. J Virol. 2021. PMID: 34110264 Free PMC article. - Identification of residues of SARS-CoV nsp1 that differentially affect inhibition of gene expression and antiviral signaling.
Jauregui AR, Savalia D, Lowry VK, Farrell CM, Wathelet MG. Jauregui AR, et al. PLoS One. 2013 Apr 29;8(4):e62416. doi: 10.1371/journal.pone.0062416. Print 2013. PLoS One. 2013. PMID: 23658627 Free PMC article. - Unique SARS-CoV protein nsp1: bioinformatics, biochemistry and potential effects on virulence.
Connor RF, Roper RL. Connor RF, et al. Trends Microbiol. 2007 Feb;15(2):51-3. doi: 10.1016/j.tim.2006.12.005. Epub 2007 Jan 4. Trends Microbiol. 2007. PMID: 17207625 Free PMC article. Review. - SARS-CoV ORF1b-encoded nonstructural proteins 12-16: replicative enzymes as antiviral targets.
Subissi L, Imbert I, Ferron F, Collet A, Coutard B, Decroly E, Canard B. Subissi L, et al. Antiviral Res. 2014 Jan;101:122-30. doi: 10.1016/j.antiviral.2013.11.006. Epub 2013 Nov 20. Antiviral Res. 2014. PMID: 24269475 Free PMC article. Review.
Cited by
- Prospective Variation of Cytokine Trends during COVID-19: A Progressive Approach from Disease Onset until Outcome.
Deus MC, Gadotti AC, Dias ES, Monte Alegre JB, Van Spitzenbergen BAK, Andrade GB, Tozoni SS, Stocco RB, Olandoski M, Tuon FFB, Pinho RA, de Noronha L, Baena CP, Moreno-Amaral AN. Deus MC, et al. Int J Mol Sci. 2024 Oct 1;25(19):10578. doi: 10.3390/ijms251910578. Int J Mol Sci. 2024. PMID: 39408907 Free PMC article. - Development and characterization of reverse genetics systems of feline infectious peritonitis virus for antiviral research.
Gu G, Fung TS, Hung WT, Osterrieder N, Go YY. Gu G, et al. Vet Res. 2024 Sep 27;55(1):124. doi: 10.1186/s13567-024-01373-z. Vet Res. 2024. PMID: 39334482 Free PMC article. - The emerging role of SARS-CoV-2 nonstructural protein 1 (nsp1) in epigenetic regulation of host gene expression.
Ivanov KI, Yang H, Sun R, Li C, Guo D. Ivanov KI, et al. FEMS Microbiol Rev. 2024 Sep 18;48(5):fuae023. doi: 10.1093/femsre/fuae023. FEMS Microbiol Rev. 2024. PMID: 39231808 Free PMC article. Review. - Binding of SARS-CoV-2 Nonstructural Protein 1 to 40S Ribosome Inhibits mRNA Translation.
Nguyen H, Nguyen HL, Li MS. Nguyen H, et al. J Phys Chem B. 2024 Jul 25;128(29):7033-7042. doi: 10.1021/acs.jpcb.4c01391. Epub 2024 Jul 15. J Phys Chem B. 2024. PMID: 39007765 Free PMC article. - Porcine deltacoronavirus nucleocapsid protein antagonizes JAK-STAT signaling pathway by targeting STAT1 through KPNA2 degradation.
Hu Y, Hao C, Wang D, Guo M, Chu H, Jin X, Zu S, Ding X, Zhang H, Hu H. Hu Y, et al. J Virol. 2024 Jul 23;98(7):e0033424. doi: 10.1128/jvi.00334-24. Epub 2024 Jun 3. J Virol. 2024. PMID: 38829137
References
- Bogdan, C. 2000. The function of type I interferons in antimicrobial immunity. Curr. Opin. Immunol. 12:419-424. - PubMed
- Cheung, C. Y., L. L. Poon, I. H. Ng, W. Luk, S. F. Sia, M. H. Wu, K. H. Chan, K. Y. Yuen, S. Gordon, Y. Guan, and J. S. Peiris. 2005. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J. Virol. 79:7819-7826. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous