Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic - PubMed (original) (raw)
Review
Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic
Kerry J Ressler et al. Nat Neurosci. 2007 Sep.
Abstract
Recent decades have witnessed tremendous advances in the neuroscience of emotion, learning and memory, and in animal models for understanding depression and anxiety. This review focuses on new rationally designed psychiatric treatments derived from preclinical human and animal studies. Nonpharmacological treatments that affect disrupted emotion circuits include vagal nerve stimulation, rapid transcranial magnetic stimulation and deep brain stimulation, all borrowed from neurological interventions that attempt to target known pathological foci. Other approaches include drugs that are given in relation to specific learning events to enhance or disrupt endogenous emotional learning processes. Imaging data suggest that common regions of brain activation are targeted with pharmacological and somatic treatments as well as with the emotional learning in psychotherapy. Although many of these approaches are experimental, the rapidly developing understanding of emotional circuit regulation is likely to provide exciting and powerful future treatments for debilitating mood and anxiety disorders.
Figures
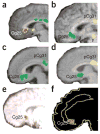
Figure 1
Subgenual cingulate cortex activation across studies. (a) Transient sadness in healthy volunteers increases activity (red) in Cg25 (arrow) measured with positron emission tomography (PET) (from ref. . Reprinted with permission from the American Journal of Psychiatry, copyright 1999, American Psychiatric Association.). (b) Decreased Cg25 activity (green) with chronic fluoxetine treatment for depression. (c) Cg25 decrease (green) in recovery with chronic fluoxetine from Parkinson’s disease–related depression. (d) Natural recovery with decreased Cg25 activity (green) in patients treated with placebo. Panels b–d reprinted from ref. by permission of Oxford University Press. (e) Predictors of response in subjects responding to CBT for depression included low pretreatment Cg25 activity (red) (from ref. . Reprinted with permission from the American Journal of Psychiatry, copyright 1999, American Psychiatric Association.). (f) Subgenual cortical decreased activity (red) was common in responders compared with nonresponders for those responding both to citalopram and to CBT for social phobia (from ref. . Reprinted from Archives of General Psychiatry, copyright 2002, American Medical Association. All rights reserved.).
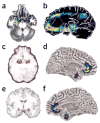
Figure 2
Amygdala activation across studies. (a) Responders compared with nonresponders showed greater decreases (red to blue indicates greatest to least decrease) in anxiety-induced amygdala activation in both citalopram and CBT treatment for social phobia (ref. . Reprinted from Archives of General Psychiatry, copyright 2002, American Medical Association. All rights reserved.). (b) In familial MDD, areas of abnormally increased cerebral blood flow (red) compared with that in controls include the amygdala (from ref. . Reprinted from Biological Psychiatry, copyright 2000, with permission from Elsevier.). (c) Predictors of response in subjects responding to CBT for depression included high sustained emotion-induced amygdala activity (circled; red indicates increased regional cerebral blood flow in response to negative words) (from ref. . Reprinted with permission from the American Journal of Psychiatry, copyright 1999, American Psychiatric Association.). (d–f) Left (d) and right (f) hemispheres showing that subgenual cingulate and amygdala show reductions in gray matter volume (red to blue indicates most to least volume decrease) in 5-HTTLPR high-risk short-allele carriers compared with homozygous long-allele genotypes (Reprinted by permission from Macmillan Publishers, Ltd.: Nature Neuroscience, ref. , copyright 2005.). Lorazepam, a benzodiazepine used for anxiety treatment, dose dependently attenuates the amygdata activation induced by emotional face viewing (e; red indicates greatest decrease in regional cerebral blood flow compared with placebo) (from ref. . Reprinted from Archives of General Psychiatry, copyright 2005, American Medical Association. All rights reserved.).
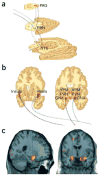
Figure 3
Ascending projections from vagus–nucleus solitarius pathways with VNS. (a) Brainstem view of ascending bilateral pathways of the central autonomic, reticular activating and limbic systems receiving afferent input from the vagus nerve. Vagal–bilateral NTS projections, through synapses in the parabrachial nuclei, project to more rostral and cortical regions. (b) Projections of NTS and parabrachial nucleus provide dense innervation of autonomic, reticular and limbic forebrain structures (from ref. . Reprinted from Neurology, by permission of Lippincott Williams & Wilkins.). NTS, nucleus of the tractus solitarius; PBN, parabrachial nucleus; PAG, periaqueductal gray matter; CNA, central nucleus of the amygdala; PVN, periventricular nucleus of the hypothalamus; VPM, ventral posteromedial nucleus of the thalamus. (c) Changes in amygdala and hippocampal regional cerebral blood flow by therapeutic vagus nerve stimulation after 4 weeks of VNS for depression (red-yellow represent areas of greatest decrease in regional cerebral blood flow relative to pretreatment values). Panels a and b reprinted from Psychiatry Research: ref. , copyright 2005, with permission from Elsevier.
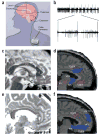
Figure 4
Deep brain stimulation. (a) Schematic of DBS used for Parkinson’s patients (modified from WebMD,
http://www.medicinenet.com/deep\_brain\_stimulation/article.htm
). (b) One possible mechanism of action of DBS-induced inhibition: recordings from a globus pallidus internus (GPi) high-frequency-discharge neuron showing inhibitory periods after each stimulus pulse (50 μA) (from ref. . Copyright 2000 by American Physiological Society. Reproduced with permission of American Physiological Society via Copyright Clearance Center.). (c) Preoperative MRI target localization for DBS for refractory depression. White dot, location of the sgCg white matter targeted for electrode placement in the DBS depression study, shown in a single subject; white arrow, sgCg gyrus; dotted line, anterior-posterior location of the electrode along the line (black) between anterior commissure and genu of the corpus callosum. (d) Baseline PET-derived blood flow: depressed patients show increased activity (red) in Cg25 compared with healthy controls. (e) Postoperative MRI in a single DBS patient showing the electrode tip in the sgCg white matter. (f) At 3 months, regions of blood flow change measured with PET in treatment responders show decreased activity (blue) compared with pretreatment. Panels c–f reprinted by permission from an article published in Neuron, ref. , copyright Elsevier 2005. CC, corpus callosum; ac, anterior commissure; g, genu of the corpus callosum; sgCg, subgenual cingulate; Cg24, cingulate area 24; sn, substantia nigra; hth, hypothalamus; mF10, medial frontal area 10; oF11, orbito-frontal area 11.
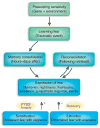
Figure 5
Emotional learning processes related to fear. The strength and regulation of emotional memories is affected by many factors both before and after the traumatic or fearful event occurs. Genetic heritability comprises up to ~40% of the risk for both depression and PTSD, and early childhood abuse is a very strong risk factor for all mood and anxiety disorders. Memories are not permanent at the time of the trauma, rather they undergo a period of consolidation in which they shift from a labile state to a more permanent state. Some evidence, at least for relatively recent memories, indicates that even after memories become consolidated, they become labile again when recalled—a process known as reconsolidation. The expression of traumatic memories, which can be the source of many symptoms in fear-related disorders, is diminished by the process of extinction, when repeated therapeutic exposures to the fear-related cues reduces or inhibits the fear memories over time. In contrast, there is some evidence that in those who develop PTSD and other pathology, a combination of avoidance of sufficient exposure with intrusive and uncontrollable memories leads to sensitization of the fear response. Arrowhead (+), sensitization increases expression of fear; filled circle (−), extinction decreases expression.
Similar articles
- Somatic treatments for mood disorders.
Rosa MA, Lisanby SH. Rosa MA, et al. Neuropsychopharmacology. 2012 Jan;37(1):102-16. doi: 10.1038/npp.2011.225. Epub 2011 Oct 5. Neuropsychopharmacology. 2012. PMID: 21976043 Free PMC article. Review. - Neuroinflammation mechanisms of neuromodulation therapies for anxiety and depression.
Guo B, Zhang M, Hao W, Wang Y, Zhang T, Liu C. Guo B, et al. Transl Psychiatry. 2023 Jan 9;13(1):5. doi: 10.1038/s41398-022-02297-y. Transl Psychiatry. 2023. PMID: 36624089 Free PMC article. Review. - [Interventional psychiatric procedures and novel substances for the treatment of affective disorders: An overview and outlook].
Bracht T, Hubl D, Adorjan K, Florineth G, Soravia LM, Draganski B, Olbrich S, Brühl A, Denier N. Bracht T, et al. Ther Umsch. 2025 Apr;82(2):57-61. doi: 10.23785/TU.2025.02.005. Ther Umsch. 2025. PMID: 40336446 Review. German. - Mood disorders in elderly population: neurostimulative treatment possibilities.
Rosenberg O, Shoenfeld N, Kotler M, Dannon PN. Rosenberg O, et al. Recent Pat CNS Drug Discov. 2009 Jun;4(2):149-59. doi: 10.2174/157488909788453013. Recent Pat CNS Drug Discov. 2009. PMID: 19519563 Review. - Deep brain stimulation: a new treatment in mood and anxiety disorders.
Velasques B, Diniz C, Teixeira S, Cartier C, Peressutti C, Silva F, de Carvalho M, Novaes A, Bittencourt J, Nardi AE, Cheniaux E, Basile L, Cagy M, Piedade R, Ribeiro P. Velasques B, et al. CNS Neurol Disord Drug Targets. 2014;13(6):961-71. doi: 10.2174/1871527313666140612122929. CNS Neurol Disord Drug Targets. 2014. PMID: 24923335 Review.
Cited by
- Altered amygdalar resting-state connectivity in depression is explained by both genes and environment.
Córdova-Palomera A, Tornador C, Falcón C, Bargalló N, Nenadic I, Deco G, Fañanás L. Córdova-Palomera A, et al. Hum Brain Mapp. 2015 Oct;36(10):3761-76. doi: 10.1002/hbm.22876. Epub 2015 Jun 19. Hum Brain Mapp. 2015. PMID: 26096943 Free PMC article. - Changes in prefrontal-limbic function in major depression after 15 months of long-term psychotherapy.
Buchheim A, Viviani R, Kessler H, Kächele H, Cierpka M, Roth G, George C, Kernberg OF, Bruns G, Taubner S. Buchheim A, et al. PLoS One. 2012;7(3):e33745. doi: 10.1371/journal.pone.0033745. Epub 2012 Mar 28. PLoS One. 2012. PMID: 22470470 Free PMC article. - TRPV1 and mast cell involvement in repeated variate stress-induced urinary bladder dysfunction in adult female mice.
Sidwell AB, Girard BM, Campbell SE, Vizzard MA. Sidwell AB, et al. Am J Physiol Renal Physiol. 2024 Sep 1;327(3):F476-F488. doi: 10.1152/ajprenal.00125.2024. Epub 2024 Jul 11. Am J Physiol Renal Physiol. 2024. PMID: 38991005 - Ketamine and the neurobiology of depression: Toward next-generation rapid-acting antidepressant treatments.
Krystal JH, Kaye AP, Jefferson S, Girgenti MJ, Wilkinson ST, Sanacora G, Esterlis I. Krystal JH, et al. Proc Natl Acad Sci U S A. 2023 Dec 5;120(49):e2305772120. doi: 10.1073/pnas.2305772120. Epub 2023 Nov 27. Proc Natl Acad Sci U S A. 2023. PMID: 38011560 Free PMC article. Review. - Cerebral Small-Vessel Disease and Risk of Incidence of Depression: A Meta-Analysis of Longitudinal Cohort Studies.
Fang Y, Qin T, Liu W, Ran L, Yang Y, Huang H, Pan D, Wang M. Fang Y, et al. J Am Heart Assoc. 2020 Aug 4;9(15):e016512. doi: 10.1161/JAHA.120.016512. Epub 2020 Jul 25. J Am Heart Assoc. 2020. PMID: 32715831 Free PMC article.
References
- Keller MB, et al. Time to recovery, chronicity, and levels of psychopathology in major depression. A 5-year prospective follow-up of 431 subjects. Arch Gen Psychiatry. 1992;49:809–816. - PubMed
- Greenberg P, et al. The economic burden of anxiety disorders in the 1990s. J Clin Psychiatry. 1999;60:427–435. - PubMed
- Gorman JM. Comorbid depression and anxiety spectrum disorders. Depress Anxiety. 1996;4:160–168. - PubMed
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12:2–19. - PubMed
Publication types
MeSH terms
Grants and funding
- R56 MH071537/MH/NIMH NIH HHS/United States
- R01 MH071537/MH/NIMH NIH HHS/United States
- MH069884/MH/NIMH NIH HHS/United States
- P50 MH58922/MH/NIMH NIH HHS/United States
- MH071537/MH/NIMH NIH HHS/United States
- DA-019624/DA/NIDA NIH HHS/United States
- P50 MH058922/MH/NIMH NIH HHS/United States
- P50 MH077083/MH/NIMH NIH HHS/United States
- 1R01MH073719/MH/NIMH NIH HHS/United States
- K01 MH069884/MH/NIMH NIH HHS/United States
- R01 MH073719/MH/NIMH NIH HHS/United States
- R01 DA019624/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical