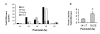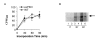PERK eIF2 alpha kinase is required to regulate the viability of the exocrine pancreas in mice - PubMed (original) (raw)
PERK eIF2 alpha kinase is required to regulate the viability of the exocrine pancreas in mice
Kaori Iida et al. BMC Cell Biol. 2007.
Abstract
Background: Deficiency of the PERK eIF2 alpha kinase in humans and mice results in postnatal exocrine pancreatic atrophy as well as severe growth and metabolic anomalies in other organs and tissues. To determine if the exocrine pancreatic atrophy is due to a cell-autonomous defect, the Perk gene was specifically ablated in acinar cells of the exocrine pancreas in mice.
Results: We show that expression of PERK in the acinar cells is required to maintain their viability but is not required for normal protein synthesis and secretion. Exocrine pancreatic atrophy in PERK-deficient mice was previously attributed to uncontrolled ER-stress followed by apoptotic cell death based on studies in cultured fibroblasts. However, we have found no evidence for perturbations in the endoplasmic reticulum or ER-stress and show that acinar cells succumb to a non-apoptotic form of cell death, oncosis, which is associated with a pronounced inflammatory response and induction of the pancreatitis stress response genes. We also show that mice carrying a knockout mutation of PERK's downstream target, ATF4, exhibit pancreatic deficiency caused by developmental defects and that mice ablated for ATF4's transcriptional target CHOP have a normal exocrine pancreas.
Conclusion: We conclude that PERK modulates secretory capacity of the exocrine pancreas by regulating cell viability of acinar cells.
Figures
Figure 1
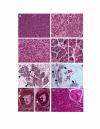
Acinar cells are lost in both PKO and exPKO mice. The PERK-deficient exocrine pancreas progressively loses acinar cells. Acinar cells are tightly packed in wild-type (A, P20). In contrast, PKO acinar cells have become degranulated giving a light pink appearance to the cytoplasm in the PKO pancreas (B, P20). The same phenotype is seen in acinar cell-specific PERK knockout (C, P19, exPKO). (D) Enlarged views of dying oncotic cells seen in (B) and (C). Some cells have lost nuclear staining (upper arrow) while others retain nuclear staining (lower arrow) (E) At P19, this particular exPKO mouse has already lost most of the exocrine pancreas although typically this degree of atrophy is not seen until 3–4 months of age. Arrows indicate examples of the smaller number of acini remaining. (F) In older mice (P162), acinar cells have been replaced by other cell types including adipocytes. Only a few dark pink acini are seen. Islets (arrows) still maintain an apparent normal structure. The animal also showed a normal glucose clearance rate. Red arrow indicates a few remaining acini. (G) In some cases, mutant acinar cells dedifferentiate into duct-like structures (P31) with abnormally large centroacinar ducts (black arrows). In the beginning of this process, duct cells still contain zymogen granules (right panel, white arrow). In some of these duct-like structures the presumptive acinar cells have completely lost zymogen granules (right panel, white arrow). (H) Conditional deletion of the Perk gene in 3-month-old CreERT2; Perk flox/flox mice also results in the appearance of oncotic cells. Two oncotic acinar cells are enlarged (inset) with lower left still exhibiting nuclear staining while the cell in the upper right shows a nuclear ghost. H&E staining. A-C, H, 200x; E, 100x; F, 80x; D, G, 600x.
Figure 6
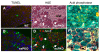
exPKO acinar cells die through oncosis. (A, B) Acinar cells in the exPKO pancreas have numerous TUNEL-positive cells (A, P31 wild-type; B, P22 exPKO). Note that the TUNEL staining pattern strikingly differs between the two genotypes. TUNEL, green; DAPI, blue; background, red. (C, D) Adjacent serial sections (3μ) of the exPKO pancreas (P19). Pale pink (oncotic) acinar cells identified by H&E staining (C) correspond to cells positive for ApoE (D); see arrows as an examples of several cells. ApoE, red; DAPI, blue; background, green. (E, F) The KO pancreas has significant leukocyte infiltration (E, wild-type; F, exPKO, P74). Dark purple acid phosphatase staining indicates infiltrated leukocytes including macrophages (see arrows) in F but not E. The background cellular staining is methylene blue.
Figure 2
The exPKO mice show pancreatitis-like phenotypes. (A) Real-time RT-PCR quantification of pancreatitis markers, Pap, Reg and Mac2, in exPKO showing induction in acinar cells at P16-32. Ratio of KO to WT is shown. Each value is either a single individual or average of multiple individuals. Variation among individuals is large due to differences in when these genes are induced and repressed. All genes were normalized to the levels of tubulin mRNA amplified from the same sample. Wilcoxon paired sample sign test and Fisher sign test for the combined data set of these four genes showed highly significant bias (P < 0.0001, Wilcoxon test and P < 0.001, Signs test) toward increased expression of these genes during the P16–P32 postnatal period. (B) The serum amylase level of the exPKO mice is significantly increased in the age group after P18 (*, Student's _t_-test, P < .01). Samples were collected from 14 and 36 exPKO animals (P14-17 and P18-25, respectively). Amylase was assayed enzymatically as described in Methods. Average fold-differences are shown (wild-type = 1.0; Error bars = s.e.m).
Figure 3
Protein synthesis in exPKO acinar cells. (A) Before the onset of massive cell death, protein synthesis is normal in the mutant pancreas. The graph shows the average of two independent experiments ([35S]Met/Cys incorporation) with a total of 4 replicates, using lobules isolated from P17 exPKO and wild-type littermates. TCA-precipitable radioactivity was normalized to the protein concentration of total lysate. (B) Protein synthesis in older exPKO mice is significantly reduced. Lobules were isolated from P63 exPKO (lanes 2, 4, 6) and wild-type (lanes 1, 3, 5) littermates and incubated for 0, 30, and 60 minutes with [35S]Met/Cys and 10 mg of total protein per lane were separated electrophoretically. The arrow indicates the position of amylase, which is the predominant zymogen in the exocrine pancreas and is readily detectable in whole radiolabeled protein. This gel was exposed for a short period and other proteins in this molecular weight range were not visible.
Figure 4
Secretion is normal in exPKO acinar cells. (A) Amylase secretion. The fraction of secreted amylase to total cellular amylase content was measured enzymatically for both basal and carbachol-stimulated pancreatic lobules. This is a representative result from 4 independent experiments. Lobules were isolated from P17 β-Perk; Perk+/+ (WT) and β-Perk; Perk-/- (PKO). The β-Perk; Perk-/-(PKO) mice are rescued for Perk in endocrine β-cells with all other tissues remaining PERK-deficient, whereas the β-Perk; Perk+/+ (WT) control mice harbor the rescuing Perk transgene in an otherwise WT background. The β-Perk; Perk-/- (PKO) mice are analogous to the exPKO mice with respect to their exocrine pancreas. Each data point represents three replicate samples. (B) Pulse-chase analysis of protein secretion. The average of two independent experiments is shown. Lobules were isolated from exPKO (PKO) and wild-type (WT) littermates (P17-18). Lobules were labeled with [35S]Met/Cys as described in Figure 3 and then chased in "cold" media over 2 hours. The fraction of TCA-precipitable radioactivity in the media to total cellular TCA-precipitable counts was calculated for each time point for both "basal," (without a secretagogues) and secretagogues-stimulated conditions ("Cch"; with 0.5 μM carbachol).
Figure 5
The ER morphology is overall normal in exPKO acinar cells. (A, B) Acinar cells of both wild-type and exPKO pancreata exhibit distended and prototypical ER at a similar frequency (A, P16 wild-type, 3000x; B, P16 exPKO, 7500x). (C) An exPKO acinar cell undergoing cell death (oncosis), P25 exPKO, 2500x. (D) The pancreas of the exPKO mouse (P25) contains infiltrating macrophages. The arrow points to the ER of an acinar cell engulfed by a macrophage. 1500x. Macrophages were not seen in the wild-type pancreas (data not shown). Also see Figure 6 for acid phosphatase staining. (E, F) Images of mutant cells undergoing cell death, 1200x. Note that these still retain the nucleus, which is vacuolated and does not contain chromatin condensation.
Figure 7
The Atf4 KO mice show pancreatic hypotrophy. As early as P4, Atf4 KO mice show fewer and smaller acinar cells in the exocrine pancreas (A, P4 wild-type; B, P4 Atf4 KO, 400x). The arrows point to tubular structures. By three months of age, the majority of the exocrine pancreas is replaced by adipocytes (C, Atf4 KO).
Similar articles
- The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas.
Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR. Zhang P, et al. Mol Cell Biol. 2002 Jun;22(11):3864-74. doi: 10.1128/MCB.22.11.3864-3874.2002. Mol Cell Biol. 2002. PMID: 11997520 Free PMC article. - Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival.
Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Harding HP, et al. Mol Cell. 2001 Jun;7(6):1153-63. doi: 10.1016/s1097-2765(01)00264-7. Mol Cell. 2001. PMID: 11430819 - Severe acute pancreatitis and reduced acinar cell apoptosis in the exocrine pancreas of mice deficient for the Cx32 gene.
Frossard JL, Rubbia-Brandt L, Wallig MA, Benathan M, Ott T, Morel P, Hadengue A, Suter S, Willecke K, Chanson M. Frossard JL, et al. Gastroenterology. 2003 Feb;124(2):481-93. doi: 10.1053/gast.2003.50052. Gastroenterology. 2003. PMID: 12557153 - Regeneration and repair of the exocrine pancreas.
Murtaugh LC, Keefe MD. Murtaugh LC, et al. Annu Rev Physiol. 2015;77:229-49. doi: 10.1146/annurev-physiol-021014-071727. Epub 2014 Oct 24. Annu Rev Physiol. 2015. PMID: 25386992 Free PMC article. Review. - New advances in pancreatic cell physiology and pathophysiology.
Weiss FU, Halangk W, Lerch MM. Weiss FU, et al. Best Pract Res Clin Gastroenterol. 2008;22(1):3-15. doi: 10.1016/j.bpg.2007.10.017. Best Pract Res Clin Gastroenterol. 2008. PMID: 18206809 Review.
Cited by
- Wolcott-Rallison syndrome.
Julier C, Nicolino M. Julier C, et al. Orphanet J Rare Dis. 2010 Nov 4;5:29. doi: 10.1186/1750-1172-5-29. Orphanet J Rare Dis. 2010. PMID: 21050479 Free PMC article. Review. - Pancreatic adaptive responses in alcohol abuse: Role of the unfolded protein response.
Lugea A, Waldron RT, Pandol SJ. Lugea A, et al. Pancreatology. 2015 Jul;15(4 Suppl):S1-5. doi: 10.1016/j.pan.2015.01.011. Epub 2015 Feb 7. Pancreatology. 2015. PMID: 25736240 Free PMC article. Review. - Frequency and spectrum of Wolcott-Rallison syndrome in Saudi Arabia: a systematic review.
Habeb AM. Habeb AM. Libyan J Med. 2013 Jun 10;8(1):21137. doi: 10.3402/ljm.v8i0.21137. Libyan J Med. 2013. PMID: 23759358 Free PMC article. Review. - Novel mutation in wolcott-rallison syndrome with variable expression in two omani siblings.
Al-Sinani S, Al-Yaarubi S, Sharef SW, Al-Murshedi F, Al-Maamari W. Al-Sinani S, et al. Oman Med J. 2015 Mar;30(2):138-41. doi: 10.5001/omj.2015.29. Oman Med J. 2015. PMID: 25960841 Free PMC article. - Acute ablation of PERK results in ER dysfunctions followed by reduced insulin secretion and cell proliferation.
Feng D, Wei J, Gupta S, McGrath BC, Cavener DR. Feng D, et al. BMC Cell Biol. 2009 Sep 4;10:61. doi: 10.1186/1471-2121-10-61. BMC Cell Biol. 2009. PMID: 19732428 Free PMC article.
References
- Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002;22:3864–3874. doi: 10.1128/MCB.22.11.3864-3874.2002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK62049/DK/NIDDK NIH HHS/United States
- R01 GM056957/GM/NIGMS NIH HHS/United States
- GM56957/GM/NIGMS NIH HHS/United States
- R56 GM056957/GM/NIGMS NIH HHS/United States
- R01 DK062049/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials