Axonal netrin-Gs transneuronally determine lamina-specific subdendritic segments - PubMed (original) (raw)
Axonal netrin-Gs transneuronally determine lamina-specific subdendritic segments
Sachiko Nishimura-Akiyoshi et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Axons from a distinct group of neurons make contact with dendritic trees of target neurons in clearly segregated and laminated patterns, thereby forming functional units for processing multiple inputs of information in the vertebrate central nervous system. Whether and how dendrites acquire lamina-specific properties corresponding to each pathway is not known. We show here that vertebrate-specific membrane-anchored members of the UNC-6/netrin family, netrin-G1 and -G2, organize the lamina/pathway-specific differentiation of dendrites. Netrin-G1 and -G2 distribute on axons of different pathways and specifically interact with receptors NGL-1 and -2, respectively. In the hippocampus, parietal cortex, and piriform cortex, NGL-1 is concentrated in the dendritic segments corresponding to the lamina-specific termination of netrin-G1-positive axons, and NGL-2 is concentrated in distinct dendritic segments corresponding to the termination of netrin-G2-positive axons. In netrin-G1- and -G2-deficient mice, in which axonal path-finding is normal, the segmental distribution of NGL-1 and -2 is selectively disrupted, and the individual receptors are diffused along the dendrites. These findings indicate that transneuronal interactions of netrin-Gs and their specific receptors provide a molecular basis for the axonal innervation-dependent mechanism of postsynaptic membrane organization, and provide insight into the formation of the laminar structure within the dendrites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
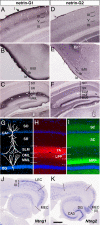
Fig. 1.
Selective distribution of netrin-G1 and -G2 proteins in distinct pathways. Coronal sections of adult mouse brain were stained with anti-netrin-G1 (A–C) and anti-netrin-G2 antibodies (D–F). Netrin-G1 and -G2 had a nonoverlapping and layer-specific distribution in the neocortex, piriform cortex, and hippocampus. Laminar organization (G), and distribution of netrin-G1 (H) and netrin-G2 (I) in the hippocampus. Netrin-G1 immunoreactivity was restricted to the terminal layers of the lateral perforant paths (LPP) in the DG and TA in CA1, whereas netrin-G2 was restricted to the terminal layers of the medial perforant paths (MPP) and SC. En, endopiriform nucleus; MML, middle molecular layer; OML, outer molecular layer; SLM, stratum lacunosum moleculare; SO, stratum oriens; SR, stratum radiatum. (J and K) Serial horizontal sections were hybridized with digoxigenin-labeled RNA probes. Note the complementary expression of Ntng1 and Ntng2 mRNAs in the LEC and MEC. [Scale bar: 200 μm (A and D); 250 μm (B, C, E, and F); 140 μm (G–I); 500 μm (J and K).]
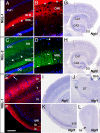
Fig. 2.
Differential binding of netrin-G1 and -G2 to the NGL family proteins. Recombinant myc-tagged proteins of mouse netrin-G1 and -G2 were added to the HEK293T cells expressing NGLs. Surface binding of ligands was immunocytochemically detected with anti-myc antibody. Netrin-G1 specifically bound to NGL-1 (A), but not NGL-2-expressing cells (B). In contrast, netrin-G2 bound to NGL-2-expressing cells (D) without affinity to NGL-1 (C) (Scale bar: 20 μm). Binding of serially diluted netrin-G1 (E) and netrin-G2 (F) proteins to the sensorchip derivatized with NGL proteins was monitored by BIAcore biosensor, and affinities (_K_d) of the interaction were determined.
Fig. 3.
Selective distribution of NGL-1 and -2 to distinct dendritic segments. Coronal sections of adult mouse brain were stained with anti-NGL-1 (A, B, E, and F) and anti-NGL-2 antibodies (C and D). NGL-1 proteins were detected in specific layers of the hippocampus [A and B (magnified image of the DG)], neocortex (E), and piriform cortex (F) with patterns similar to those of netrin-G1. (C) The lamina-selective distribution of NGL-2 proteins in hippocampus matches that of netrin-G2. (D) Dendritic staining of NGL-2 (arrowheads) at high magnification. Asterisks indicate the hippocampal fissure. (G and H) In situ hybridization analysis of horizontal brain sections revealed high expression levels of Ngl1 and Ngl2 mRNAs in pyramidal neurons of CA1-CA3 and granule cells of the DG. (I–L) Expression sites of Ngl1 in other regions. Ngl1 transcripts were abundant in the neocortex (I) and piriform cortex (K), but very low in the dorsal thalamus (DT; J) and olfactory bulb (L). MHb, medial habenular nucleus; Mi, mitral cell layer; Rt, reticular thalamic nucleus. [Scale bar: 200 μm (A, C, E, and F); 50 μm (B and D); 690 μm (G and H); 750 μm (I and J); 605 μm (K); 820 μm (L).]
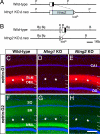
Fig. 4.
Targeted disruption of netrin-G1 and -G2 genes. Schematic representation of the wild-type and mutated locus of the Ntng1 (A) and Ntng2 genes (B). Open boxes denote the 5′ UTR and filled boxes indicate the coding region. The pgk-neo cassette flanked by loxP sequences was excised by crossing with transgenic mice expressing Cre recombinase (Δneo). B, BamHI; Bg, BglII; K, KpnI; P, PstI. Immunohistochemistry of netrin-G1 (C–E) and netrin-G2 (F–H) in the hippocampus revealed null mutation of the targeted netrin-G and no compensatory expression of the other in each mutant line. (Scale bar: 200 μm.)
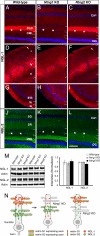
Fig. 5.
Loss of lamina-restricted distribution of NGLs in netrin-G-deficient mice. Distribution patterns of NGL-1 (A–I) and NGL-2 (J–L) in wild-type and netrin-G mutant mice are shown by immunohistochemistry. Lamina-specific staining of NGL-1 was absent in Ntng1 KO hippocampus (B), neocortex layer I (E), and piriform cortex (H). Similarly, there was no laminar distribution of NGL-2 in Ntng2 KO hippocampus. Note the molecular layer of the DG, which is evenly stained with NGL-2 (L). Asterisks indicate the hippocampal fissure. [Scale bar: 200 μm (A–F and J–L); 250 μm (G–I).] (M) Western blot analysis of NGLs in hippocampus of wild-type, Ntng1 KO, and Ntng2 KO mice (n = 4 for each genotype) did not reveal any significant differences in the receptor protein levels, indicating a mislocation of, but not a decrease in, receptors in the mutant hippocampus. Data are presented as mean ± SEM. (N) Model for transneuronal regulation of receptor localization by axonal netrin-G proteins. Distinct axon populations, each expressing netrin-G1 or -G2, make contact with distinct segments of dendrites of target neurons. NGL-1 and -2, which are expressed in the target neurons, are anchored by axon-derived netrin-G1 and -G2, and are thereby precisely arranged onto the selected subdendritic segments. Without netrin-Gs as extrinsic cues, NGLs would be dispersed across multiple segments of dendrites.
Similar articles
- Netrin-G/NGL complexes encode functional synaptic diversification.
Matsukawa H, Akiyoshi-Nishimura S, Zhang Q, Luján R, Yamaguchi K, Goto H, Yaguchi K, Hashikawa T, Sano C, Shigemoto R, Nakashiba T, Itohara S. Matsukawa H, et al. J Neurosci. 2014 Nov 19;34(47):15779-92. doi: 10.1523/JNEUROSCI.1141-14.2014. J Neurosci. 2014. PMID: 25411505 Free PMC article. - The netrin-G1 ligand NGL-1 promotes the outgrowth of thalamocortical axons.
Lin JC, Ho WH, Gurney A, Rosenthal A. Lin JC, et al. Nat Neurosci. 2003 Dec;6(12):1270-6. doi: 10.1038/nn1148. Epub 2003 Nov 2. Nat Neurosci. 2003. PMID: 14595443 - Netrin-1 directs dendritic growth and connectivity of vertebrate central neurons in vivo.
Nagel AN, Marshak S, Manitt C, Santos RA, Piercy MA, Mortero SD, Shirkey-Son NJ, Cohen-Cory S. Nagel AN, et al. Neural Dev. 2015 Jun 10;10:14. doi: 10.1186/s13064-015-0041-y. Neural Dev. 2015. PMID: 26058786 Free PMC article. - The NGL family of leucine-rich repeat-containing synaptic adhesion molecules.
Woo J, Kwon SK, Kim E. Woo J, et al. Mol Cell Neurosci. 2009 Sep;42(1):1-10. doi: 10.1016/j.mcn.2009.05.008. Epub 2009 May 23. Mol Cell Neurosci. 2009. PMID: 19467332 Review. - The netrin protein family.
Rajasekharan S, Kennedy TE. Rajasekharan S, et al. Genome Biol. 2009;10(9):239. doi: 10.1186/gb-2009-10-9-239. Epub 2009 Sep 29. Genome Biol. 2009. PMID: 19785719 Free PMC article. Review.
Cited by
- Modification of temporal pattern sensitivity for inputs from medial entorhinal cortex by lateral inputs in hippocampal granule cells.
Nakajima N, Kamijo T, Hayakawa H, Sugisaki E, Aihara T. Nakajima N, et al. Cogn Neurodyn. 2024 Jun;18(3):1047-1059. doi: 10.1007/s11571-023-09964-w. Epub 2023 Apr 13. Cogn Neurodyn. 2024. PMID: 38826655 - Axon-axon interactions determine modality-specific wiring and subcellular synaptic specificity in a somatosensory circuit.
Galindo SE, Wood AJ, Cooney PC, Hammond LA, Grueber WB. Galindo SE, et al. Development. 2023 Mar 1;150(5):dev199832. doi: 10.1242/dev.199832. Epub 2023 Mar 15. Development. 2023. PMID: 36920224 Free PMC article. - A Proteome-Wide Effect of PHF8 Knockdown on Cortical Neurons Shows Downregulation of Parkinson's Disease-Associated Protein Alpha-Synuclein and Its Interactors.
Oey NE, Zhou L, Chan CHS, VanDongen AMJ, Tan EK. Oey NE, et al. Biomedicines. 2023 Feb 8;11(2):486. doi: 10.3390/biomedicines11020486. Biomedicines. 2023. PMID: 36831023 Free PMC article. - A role for axon-glial interactions and Netrin-G1 signaling in the formation of low-threshold mechanoreceptor end organs.
Meltzer S, Boulanger KC, Osei-Asante E, Handler A, Zhang Q, Sano C, Itohara S, Ginty DD. Meltzer S, et al. Proc Natl Acad Sci U S A. 2022 Oct 25;119(43):e2210421119. doi: 10.1073/pnas.2210421119. Epub 2022 Oct 17. Proc Natl Acad Sci U S A. 2022. PMID: 36252008 Free PMC article. - Mechanisms Underlying Target Selectivity for Cell Types and Subcellular Domains in Developing Neocortical Circuits.
Gutman-Wei AY, Brown SP. Gutman-Wei AY, et al. Front Neural Circuits. 2021 Sep 24;15:728832. doi: 10.3389/fncir.2021.728832. eCollection 2021. Front Neural Circuits. 2021. PMID: 34630048 Free PMC article. Review.
References
- Sanes JR, Yamagata M. Curr Opin Neurobiol. 1999;9:79–87. - PubMed
- Witter MP. Hippocampus. 1993;3:33–44. - PubMed
- Skutella T, Nitsch R. Trends Neurosci. 2001;24:107–113. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous