Affinity thresholds for membrane fusion triggering by viral glycoproteins - PubMed (original) (raw)
Affinity thresholds for membrane fusion triggering by viral glycoproteins
Kosei Hasegawa et al. J Virol. 2007 Dec.
Abstract
Enveloped viruses trigger membrane fusion to gain entry into cells. The receptor affinities of their attachment proteins vary greatly, from 10(-4) M to 10(-9) M, but the significance of this is unknown. Using six retargeted measles viruses that bind to Her-2/neu with a 5-log range in affinity, we show that receptor affinity has little impact on viral attachment but is nevertheless a key determinant of infectivity and intercellular fusion. For a given cell surface receptor density, there is an affinity threshold above which cell-cell fusion proceeds efficiently. Suprathreshold affinities do not further enhance the efficiency of membrane fusion.
Figures
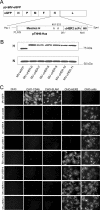
FIG. 1.
Characterization of the HER2/neu-retargeted measles viruses. (A) Schematic representation of the HER2-retargeted viruses. The Y481A and R533A mutations ablate CD46 and SLAM interactions. The scFv was inserted as a SfiI/NotI fragment and displayed as a C-terminal extension on mutated HA proteins with a His6 tag. (B) Immunoblotting results of the parental and HER2-retargeted virions using anti-HA and anti-N antibodies. Equal titers of each virus were loaded. (C) Specificity of the receptor usage of parental and fully retargeted anti-HER2 measles viruses. CHO cells and CHO cells stably expressing CD46, SLAM, HER2, or αHis were infected with the parental MV-eGFP, the CD38-retargeted, or the HER2-retargeted measles viruses at an MOI of 1.0. Cells were photographed with fluorescence microscopy after 48 h.
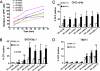
FIG. 2.
The impact of receptor affinity on virus binding and infectivity. (A) There is no correlation between receptor affinity and virus binding with SKOV3ip.1 cells. HER2-retargeted viruses were allowed to bind to SKOV3ip.1 cells on ice for various times, after which unbound viruses were removed. Numbers of GFP-positive cells were counted at 48 h postinfection and plotted. The negative control virus MV-αCD38 displays a CD38-specific scFv and does not bind HER2/neu. (B, C, and D) We next determined the impact of receptor affinity on viral infectivity with high-HER2-expressing SKOV3ip.1 cells or low-HER2-expressing TE671 cells (40-fold fewer HER2 receptors). CHO cells expressing the pseudoreceptor αHis were used as a positive control for virus infectivity. Cells were infected at an MOI of 1.0 or 4.0, and the numbers of GFP-positive cells were counted by flow cytometry at 48 h postinfection. Error bars represent standard deviations. P value was determined by one-way analysis of variance at an MOI of 4.0. Asterisks (**) indicate a significant difference (P < 0.01) between the test group and the MV-αHER-8-infected cells, using Dunnett's multiple comparison test.
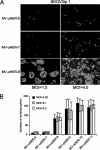
FIG. 3.
Influence of MOI on syncytial formation of low- and high-affinity viruses. (A) HER2-overexpressing SKOV3ip.1 cells were infected at MOIs of 1.0 and 4.0 with retargeted viruses. Photographs were taken at 72 h postinfection, and syncytial formations were compared. Increasing the numbers of infectious centers by increasing MOI did not result in syncytial formation for the low-affinity viruses. (B) SKOV3ip.1 cells were infected with each member of the HER2-retargeted virus panel at MOIs of 0.02, 0.1, and 0.5. Cells were fixed and stained with Hoechst 33342 at 72 h postinfection. Numbers of nuclei in each syncytium were counted (10 to 20 syncytia were counted). Sizes of the syncytia were not affected by MOI and were comparable at all MOIs.
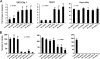
FIG. 4.
Cytopathic potential of the HER2-retargeted measles viruses. HER2-overexpressing SKOV3ip.1 cells, low-HER2-expressing TE671 cells, and Vero-αHis cells were infected with HER2-retargeted viruses at an MOI of 1.0. Virus-induced apoptosis (A) and antiproliferative effects (B) were analyzed by enzyme-linked immunosorbent assay and by clonogenic assay, respectively. Error bars represent ± standard deviations. The P value was determined by one-way analysis of variance. Asterisks (**) indicate a significant difference (P < 0.01) between the test group and the MV-αHER-8-infected group, using Dunnett's multiple comparison test.
FIG. 5.
The impact of HER2 receptor density and affinity with virus-induced cell-to-cell fusion. (A) A panel of tumor cell lines with various amounts of HER2 receptors was infected with each of the HER2-retargeted viruses at an MOI of 1.0. Asterisks (*) denote receptor levels below the levels of detection (103). Photographs were taken at 72 h postinfection. (B) CHO cells or CHO clones expressing low and high HER2 receptors and CHO-αHis cells were treated as shown in panel A.
Similar articles
- The receptor attachment function of measles virus hemagglutinin can be replaced with an autonomous protein that binds Her2/neu while maintaining its fusion-helper function.
Rasbach A, Abel T, Münch RC, Boller K, Schneider-Schaulies J, Buchholz CJ. Rasbach A, et al. J Virol. 2013 Jun;87(11):6246-56. doi: 10.1128/JVI.03298-12. Epub 2013 Mar 27. J Virol. 2013. PMID: 23536664 Free PMC article. - Targeted entry of enveloped viruses: measles and herpes simplex virus I.
Navaratnarajah CK, Miest TS, Carfi A, Cattaneo R. Navaratnarajah CK, et al. Curr Opin Virol. 2012 Feb;2(1):43-9. doi: 10.1016/j.coviro.2011.12.002. Epub 2011 Dec 23. Curr Opin Virol. 2012. PMID: 22440965 Free PMC article. Review. - Model of the TVA receptor determinants required for efficient infection by subgroup A avian sarcoma and leukosis viruses.
Melder DC, Pike GM, VanBrocklin MW, Federspiel MJ. Melder DC, et al. J Virol. 2015 Feb;89(4):2136-48. doi: 10.1128/JVI.02339-14. Epub 2014 Dec 3. J Virol. 2015. PMID: 25473063 Free PMC article. - Nectin-2-mediated entry of a syncytial strain of herpes simplex virus via pH-independent fusion with the plasma membrane of Chinese hamster ovary cells.
Delboy MG, Patterson JL, Hollander AM, Nicola AV. Delboy MG, et al. Virol J. 2006 Dec 27;3:105. doi: 10.1186/1743-422X-3-105. Virol J. 2006. PMID: 17192179 Free PMC article. - Structural and mechanistic studies of measles virus illuminate paramyxovirus entry.
Plemper RK, Brindley MA, Iorio RM. Plemper RK, et al. PLoS Pathog. 2011 Jun;7(6):e1002058. doi: 10.1371/journal.ppat.1002058. Epub 2011 Jun 2. PLoS Pathog. 2011. PMID: 21655106 Free PMC article. Review.
Cited by
- Measles to the Rescue: A Review of Oncolytic Measles Virus.
Aref S, Bailey K, Fielding A. Aref S, et al. Viruses. 2016 Oct 22;8(10):294. doi: 10.3390/v8100294. Viruses. 2016. PMID: 27782084 Free PMC article. Review. - DARPin-targeting of measles virus: unique bispecificity, effective oncolysis, and enhanced safety.
Friedrich K, Hanauer JR, Prüfer S, Münch RC, Völker I, Filippis C, Jost C, Hanschmann KM, Cattaneo R, Peng KW, Plückthun A, Buchholz CJ, Cichutek K, Mühlebach MD. Friedrich K, et al. Mol Ther. 2013 Apr;21(4):849-59. doi: 10.1038/mt.2013.16. Epub 2013 Feb 5. Mol Ther. 2013. PMID: 23380817 Free PMC article. - Advances in the design and development of oncolytic measles viruses.
Hutzen B, Raffel C, Studebaker AW. Hutzen B, et al. Oncolytic Virother. 2015 Aug 27;4:109-18. doi: 10.2147/OV.S66078. eCollection 2015. Oncolytic Virother. 2015. PMID: 27512675 Free PMC article. Review. - Probing Morbillivirus Antisera Neutralization Using Functional Chimerism between Measles Virus and Canine Distemper Virus Envelope Glycoproteins.
Muñoz-Alía MA, Russell SJ. Muñoz-Alía MA, et al. Viruses. 2019 Jul 27;11(8):688. doi: 10.3390/v11080688. Viruses. 2019. PMID: 31357579 Free PMC article. - Structural and functional studies on the interaction of adenovirus fiber knobs and desmoglein 2.
Wang H, Yumul R, Cao H, Ran L, Fan X, Richter M, Epstein F, Gralow J, Zubieta C, Fender P, Lieber A. Wang H, et al. J Virol. 2013 Nov;87(21):11346-62. doi: 10.1128/JVI.01825-13. Epub 2013 Aug 14. J Virol. 2013. PMID: 23946456 Free PMC article.
References
- Adams, G. P., M. S. Tai, J. E. McCartney, J. D. Marks, W. F. Stafford III, L. L. Houston, J. S. Huston, and L. M. Weiner. 2006. Avidity-mediated enhancement of in vivo tumor targeting by single-chain Fv dimers. Clin. Cancer Res. 12:1599-1605. - PubMed
- Anderson, B. D., T. Nakamura, S. J. Russell, and K. W. Peng. 2004. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 64:4919-4926. - PubMed
- Bookman, M. A., K. M. Darcy, D. Clarke-Pearson, R. A. Boothby, and I. R. Horowitz. 2003. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. J. Clin. Oncol. 21:283-290. - PubMed
- Dingli, D., K. W. Peng, M. E. Harvey, P. R. Greipp, M. K. O'Connor, R. Cattaneo, J. C. Morris, and S. J. Russell. 2004. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood 103:1641-1646. - PubMed
- Dorig, R. E., A. Marcil, A. Chopra, and C. D. Richardson. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75:295-305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous