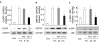deltaPKC participates in the endoplasmic reticulum stress-induced response in cultured cardiac myocytes and ischemic heart - PubMed (original) (raw)
deltaPKC participates in the endoplasmic reticulum stress-induced response in cultured cardiac myocytes and ischemic heart
Xin Qi et al. J Mol Cell Cardiol. 2007 Oct.
Abstract
The cellular response to excessive endoplasmic reticulum (ER) stress includes the activation of signaling pathways, which lead to apoptotic cell death. Here we show that treatment of cultured cardiac myocytes with tunicamycin, an agent that induces ER stress, causes the rapid translocation of deltaPKC to the ER. We further demonstrate that inhibition of deltaPKC using the deltaPKC-specific antagonist peptide, deltaV1-1, reduces tunicamycin-induced apoptotic cell death, and inhibits expression of specific ER stress response markers such as CHOP, GRP78 and phosphorylation of JNK. The physiological importance of deltaPKC in this event is further supported by our findings that the ER stress response is also induced in hearts subjected to ischemia and reperfusion injury and that this response also involves deltaPKC translocation to the ER. We found that the levels of the ER chaperone, GRP78, the spliced XBP-1 and the phosphorylation of JNK are all increased following ischemia and reperfusion and that deltaPKC inhibition by deltaV1-1 blocks these events. Therefore, ischemia-reperfusion injury induces ER stress in the myocardium in a mechanism that requires deltaPKC activity. Taken together, our data show for the first time that deltaPKC activation plays a critical role in the ER stress-mediated response and the resultant cell death.
Figures
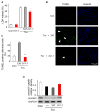
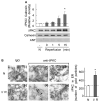
Fig 1. δPKC activation modulates tunicamycin-induced cell death and apoptosis in cardiac myocytes
A. Cell death after Tm-treatment (3μg/ml, 40 hours) was measured by LDH release in the presence of the δPKC antagonist peptide, δV1-1, or the TAT control peptide (1 μM). Data are mean ± S.E of four independent experiments. Student t test; * p<0.05 vs. Tm treatment. B. Untreated or tunicamycin-treated cardiac myocytes (3 μg/ml, 24 hours) in the presence of control peptide (Tm+TAT) or the δPKC inhibitor, δV1-1 (Tm + δV1-1) were co-stained with the TUNEL method, to identify apoptotic cells, and with the nuclear dye, Hoechst, to identify the cells in the field. Arrows point out some of the TUNEL-positive cells. C. TUNEL-positive cells were counted in a total of more than 300 myocytes over 3 random fields and expressed as percentages of the total number of nuclei. Data are expressed as mean ± S.E. of three independent experiments. Student t test; ** p<0.01 vs. Tm treatment. D. δV1-1 inhibits caspase-3 activation induced by Tm treatment. Procaspase-3 was detected 24 hours after Tm treatment. The data are expressed as mean ± S.E of three independent experiments.
Fig 2. δPKC activation regulates signals triggered by tunicamycin-induced ER stress in cardiac myocytes
After 6 hours (A) and 24 hours (B, C) of Tm treatment (3μg/ml) in the presence of TAT or δV1-1, total lysates of myocytes were analyzed by Western blot for the presence of the pro-apoptotic ER stress-induced protein, CHOP (A), the ER chaperone, GRP78 (B) and phosphorylation of the apoptotic kinase, JNK (C). Shown are representative blots of three independent experiments. Data are expressed as mean ± S.E. Student t test; * p < 0.05 vs. Tm treatment. GAPDH and JNK were used as internal normalization standards.
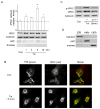
Fig 3. δPKC translocates to the ER of Tm-treated cardiac myocytes
A. ER fractions from Tm-treated myocytes were subjected to Western blot analysis with an anti-δPKC antibody. The ER-specific calnexin protein was used as an internal loading control for normalization. Purity of ER fractions was confirmed using the mitochondrial marker, ANT, and the cytoplasmic marker, enolase. Data represent mean ± S.E. of three independent experiments. Student t test; * p<0.05 vs. no treatment (Con). B. Representative confocal pictures of δPKC (red) and PDI (green, an ER marker) demonstrating increased co-localization (yellow) in Tm-treated cardiac myocytes. Data are representative of three independent experiments. C. ER fractions from cells treated with Tm in the presence of δV1-1 or TAT were subjected to Western blot with an anti-Phospho-Ser643-δPKC (p-δPKC, a marker of active δPKC) antibody. An anti-δPKC antibody or anti-calnexin were used as internal controls for normalization. D. The ER, mitochondria and cytosol were probed with the fraction specific markers, calnexin, ANT and enolase, respectively.
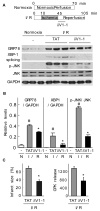
Fig 4. δPKC modulates the ER stress response of the myocardium in a model of cardiac ischemia and reperfusion injury
A. Normoxic control hearts and hearts that underwent ischemia and reperfusion were homogenized and total extracts were isolated. The levels of GRP78, spliced XBP1 and phospho-JNK were determined by Western blot. B. Quantitative data of the hearts described in (A). Values represent mean ± S.E. of three animals in each group (N: normoxia; I/R: ischemia/reperfusion). Student t test; * p<0.05 vs. TAT treatment, # p< 0.05 vs. control. C. Hearts were subjected to ischemia-reperfusion and treated at the onset of reperfusion with TAT control peptide or δV1-1 and the infarct size (left panel) and cell survival, as demonstrated by the decrease in CPK release (right panel), were determined. Data are expressed as mean ± S.E. of three animals in each group. Student t test; * p<0.05 vs. TAT treatment.
Fig 5. δPKC translocates to the ER in an ex vivo model of ischemia-reperfusion injury
A. ER fractions of normoxic and ischemia-reperfusion injured hearts were subjected to Western blot analysis with an anti-δPKC antibody. Whereas ischemia (0 min reperfusion) did not induce translocation of δPKC to the ER as compared with normoxic control hearts (N), reperfusion significantly induced δPKC translocation to the ER by 5 minutes. Shown are representative Western blots (bottom) and a histogram depicting the amount of δPKC associated with the ER in heart samples (top). δPKC levels were normalized to the ER marker, calnexin. Purity of the ER fractions was confirmed by the lack of the mitochondrial marker, ANT. Student t test; * p < 0.05 vs. control normoxia. B. ER localization of δPKC as evidenced by immuno-electron microscopy. Representative electron micrographs of δPKC staining in the ER fractions from normoxic control hearts (N) and hearts subjected to 35 min of ischemia followed by 15 min of reperfusion (I/R). (magnification: × 35000). Samples were probed in the presence (+) or absence (-) of δPKC antibody. Arrows indicate δPKC-positive staining with gold particles. Quantitative data of gold particles associated with ER lumen are provided in the right histogram. Five random fields of each section from two animals were counted. The data represent mean ± SD of two animals in each group. Student t test; * p < 0.05 vs. control normoxia.
Similar articles
- Impaired perfusion after myocardial infarction is due to reperfusion-induced deltaPKC-mediated myocardial damage.
Ikeno F, Inagaki K, Rezaee M, Mochly-Rosen D. Ikeno F, et al. Cardiovasc Res. 2007 Mar 1;73(4):699-709. doi: 10.1016/j.cardiores.2006.12.011. Epub 2006 Dec 13. Cardiovasc Res. 2007. PMID: 17234167 Free PMC article. - Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis.
Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, Yutani C, Ozawa K, Ogawa S, Tomoike H, Hori M, Kitakaze M. Okada K, et al. Circulation. 2004 Aug 10;110(6):705-12. doi: 10.1161/01.CIR.0000137836.95625.D4. Epub 2004 Aug 2. Circulation. 2004. PMID: 15289376 - Ischemic postconditioning protects myocardium from ischemia/reperfusion injury through attenuating endoplasmic reticulum stress.
Liu XH, Zhang ZY, Sun S, Wu XD. Liu XH, et al. Shock. 2008 Oct;30(4):422-7. doi: 10.1097/SHK.0b013e318164ca29. Shock. 2008. PMID: 18323739 - Globular adiponectin attenuates myocardial ischemia/reperfusion injury by upregulating endoplasmic reticulum Ca²⁺-ATPase activity and inhibiting endoplasmic reticulum stress.
Guo J, Bian Y, Bai R, Li H, Fu M, Xiao C. Guo J, et al. J Cardiovasc Pharmacol. 2013 Aug;62(2):143-53. doi: 10.1097/FJC.0b013e31829521af. J Cardiovasc Pharmacol. 2013. PMID: 23609327 - Endoplasmic reticulum stress: cell life and death decisions.
Xu C, Bailly-Maitre B, Reed JC. Xu C, et al. J Clin Invest. 2005 Oct;115(10):2656-64. doi: 10.1172/JCI26373. J Clin Invest. 2005. PMID: 16200199 Free PMC article. Review.
Cited by
- Protective effect of Barbaloin in a rat model of myocardial ischemia reperfusion injury through the regulation of the CNPY2‑PERK pathway.
Cui Y, Wang Y, Liu G. Cui Y, et al. Int J Mol Med. 2019 May;43(5):2015-2023. doi: 10.3892/ijmm.2019.4123. Epub 2019 Mar 5. Int J Mol Med. 2019. PMID: 30864682 Free PMC article. - Novel roles for protein kinase Cdelta-dependent signaling pathways in acute hypoxic stress-induced autophagy.
Chen JL, Lin HH, Kim KJ, Lin A, Forman HJ, Ann DK. Chen JL, et al. J Biol Chem. 2008 Dec 5;283(49):34432-44. doi: 10.1074/jbc.M804239200. Epub 2008 Oct 3. J Biol Chem. 2008. PMID: 18836180 Free PMC article. - Mitochondrial reactive oxygen species at the heart of the matter: new therapeutic approaches for cardiovascular diseases.
Kornfeld OS, Hwang S, Disatnik MH, Chen CH, Qvit N, Mochly-Rosen D. Kornfeld OS, et al. Circ Res. 2015 May 22;116(11):1783-99. doi: 10.1161/CIRCRESAHA.116.305432. Circ Res. 2015. PMID: 25999419 Free PMC article. Review. - Calcium-sensing receptor activating phosphorylation of PKCδ translocation on mitochondria to induce cardiomyocyte apoptosis during ischemia/reperfusion.
Zheng H, Liu J, Liu C, Lu F, Zhao Y, Jin Z, Ren H, Leng X, Jia J, Hu G, Dong S, Zhong X, Li H, Yang B, Xu C, Zhang W. Zheng H, et al. Mol Cell Biochem. 2011 Dec;358(1-2):335-43. doi: 10.1007/s11010-011-0984-1. Epub 2011 Jul 16. Mol Cell Biochem. 2011. PMID: 21766206 - Schisandrin B Ameliorates Myocardial Ischemia/Reperfusion Injury Through Attenuation of Endoplasmic Reticulum Stress-Induced Apoptosis.
Zhang W, Sun Z, Meng F. Zhang W, et al. Inflammation. 2017 Dec;40(6):1903-1911. doi: 10.1007/s10753-017-0631-4. Inflammation. 2017. PMID: 28748322
References
- Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 2006;13:363–73. - PubMed
- Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–9. - PubMed
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. - PubMed
- Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res. 2006;99:275–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL076675/HL/NHLBI NIH HHS/United States
- R01 HL076675-03/HL/NHLBI NIH HHS/United States
- R01 HL076675-04/HL/NHLBI NIH HHS/United States
- HL76675/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous