ELYS/MEL-28 chromatin association coordinates nuclear pore complex assembly and replication licensing - PubMed (original) (raw)
ELYS/MEL-28 chromatin association coordinates nuclear pore complex assembly and replication licensing
Peter J Gillespie et al. Curr Biol. 2007.
Abstract
Xenopus egg extract supports all the major cell-cycle transitions in vitro. We have used a proteomics approach to identify proteins whose abundance on chromatin changes during the course of an in vitro cell cycle. One of the proteins we identified was ELYS/MEL-28, which has recently been described as the earliest-acting factor known to be required for nuclear pore complex (NPC) assembly [1-4]. ELYS interacts with the Nup107-160 complex and is required for its association with chromatin. ELYS contains an AT-hook domain, which we show binds to chromatin with a high affinity. This domain can compete with full-length ELYS for chromatin association, thereby blocking NPC assembly. This provides evidence that ELYS interacts directly with chromatin and that this interaction is essential for NPC assembly and compartmentalization of chromosomal DNA within the cell. Furthermore, we detected a physical association on chromatin between ELYS and the Mcm2-7 replication-licensing proteins. ELYS chromatin loading, NPC assembly, and nuclear growth were delayed when Mcm2-7 was prevented from loading onto chromatin. Because nuclear assembly is required to shut down licensing prior to entry into S phase, our results suggest a mechanism by which these two early cell-cycle events are coordinated with one another.
Figures
Figure 1
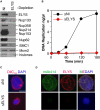
ELYS/MEL-28 Is a Chromatin-Associated Protein (A) Chromatin was isolated from egg extract at discrete time points throughout interphase and subjected to LC-MS/MS. The relative abundance of ELYS/MEL-28, polymerase δ, Mcm2, and Nup153 are indicated. The duration of S phase in this extract is indicated above the graph. (B) Sperm chromatin was incubated in either a metaphase or interphase egg extract. At the indicated times, chromatin was isolated and immunoblotted for Mcm2, SMC2, and ELYS. As a control, extract was incubated without sperm chromatin for 90 min. The lower portion of the gel was stained with Coomassie blue so that the level of histones could be determined as a control for chromatin recovery.
Figure 2
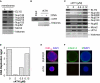
ELYS Is Required for Nuclear Pore Complex Assembly (A) Sperm chromatin was incubated in nonimmune or ELYS-depleted egg extract for 90 min. Chromatin was then isolated and immunoblotted for ELYS, Nups133, 358, 214, 153, 62, and Mcm2. The lower portion of the gel was stained with Coomassie blue so that the level of histones could be determined as a control for chromatin recovery. (B) DNA synthesis was assayed in nonimmune and ELYS-depleted extracts. (C) Sperm chromatin was incubated in nonimmune or ELYS-depleted egg extract treated with 10μg/ml DilC18 (red). Nuclei were fixed and spun onto coverslips and counterstained with DAPI (blue). The scale bar represents 10 μm. (D) Nuclei assembled in nonimmune or ELYS-depleted egg extract were fixed, spun onto coverslips, and stained with mAb414 (green) and ELYS antibodies (red) and DAPI (blue).
Figure 3
ELYS Chromatin Association Directs Nuclear Pore Complex Assembly (A) Sperm chromatin was incubated in complete or membrane-free egg extract for 60 min. Chromatin was isolated and immunoblotted for ELYS, Nups133, 358, 214, 153, 62, and Mcm2. The lower portion of the gel was stained with Coomassie blue so that the level of histones could be determined as a control for chromatin recovery. (B) rATH was incubated in the presence or absence of sperm chromatin for 30 min. Chromatin was isolated and immunoblotted for rATH. The lower portion of the gel was stained with Coomassie blue so that the level of histones could be determined as a control for chromatin recovery. Twenty-five percent of input rATH is shown as control for protein recovery. (C) Sperm chromatin was incubated for 90 min in egg extract treated with either buffer alone or rATH at the indicated concentrations. Chromatin was isolated and immunoblotted for rATH, ELYS, Nups133, 358, 214, 153, 62, and Mcm2. The lower portion of the gel was stained with Coomassie blue so that the level of histones could be determined as a control for chromatin recovery. (D) DNA synthesis was assayed in rATH-treated extracts. (E) Sperm chromatin was incubated in extract treated with 10 μg/ml DilC18 (red) and either buffer or 2 μM rATH. Nuclei were fixed and spun onto coverslips and counterstained with DAPI (blue). The scale bar represents 10 μm. (F) Nuclei assembled in egg extract treated with buffer alone or 10μM rATH were fixed, spun onto coverslips and stained with mAb414 (green) and DAPI (blue).
Figure 4
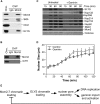
ELYS Interacts with the Replication Licensing System (A) Sperm chromatin was incubated in egg extract for 45 min. Chromatin was then isolated by centrifugation through a sucrose cushion, digested with nuclease, and immunoprecipitated with either nonimmune IgG or Mcm3 antibodies. Precipitated proteins were immunoblotted with antibodies to Mcm3, Sld5, Orc2, or ELYS. An equal sample of chromatin is shown as control for protein recovery. (B) Chromatin isolated from rATH-treated egg extract was digested with nuclease and immunoprecipiated with either nonimmune IgG or Mcm3 antibodies as in (A). Precipitated proteins were identified with antibodies to Mcm3 and rATH. (C) Sperm chromatin was incubated in egg extract treated with buffer alone or 50 nM gemininDEL. At the indicated times, chromatin was isolated and immunoblotted for ELYS, Nups133, 358, 214, 153, 62, SMC1, and Mcm2. (D) Sperm chromatin was incubated in egg extract treated with buffer alone or gemininDEL. At the indicated times, the size of randomly selected nuclei were measured. Values are shown ± the standard deviation. (E) Model for the interaction of ELYS with the replication licensing system.
Similar articles
- Capture of AT-rich chromatin by ELYS recruits POM121 and NDC1 to initiate nuclear pore assembly.
Rasala BA, Ramos C, Harel A, Forbes DJ. Rasala BA, et al. Mol Biol Cell. 2008 Sep;19(9):3982-96. doi: 10.1091/mbc.e08-01-0012. Epub 2008 Jul 2. Mol Biol Cell. 2008. PMID: 18596237 Free PMC article. - MEL-28/ELYS is required for the recruitment of nucleoporins to chromatin and postmitotic nuclear pore complex assembly.
Franz C, Walczak R, Yavuz S, Santarella R, Gentzel M, Askjaer P, Galy V, Hetzer M, Mattaj IW, Antonin W. Franz C, et al. EMBO Rep. 2007 Feb;8(2):165-72. doi: 10.1038/sj.embor.7400889. Epub 2007 Jan 19. EMBO Rep. 2007. PMID: 17235358 Free PMC article. - Nucleosomal regulation of chromatin composition and nuclear assembly revealed by histone depletion.
Zierhut C, Jenness C, Kimura H, Funabiki H. Zierhut C, et al. Nat Struct Mol Biol. 2014 Jul;21(7):617-25. doi: 10.1038/nsmb.2845. Epub 2014 Jun 22. Nat Struct Mol Biol. 2014. PMID: 24952593 Free PMC article. - ELYS is a dual nucleoporin/kinetochore protein required for nuclear pore assembly and proper cell division.
Rasala BA, Orjalo AV, Shen Z, Briggs S, Forbes DJ. Rasala BA, et al. Proc Natl Acad Sci U S A. 2006 Nov 21;103(47):17801-6. doi: 10.1073/pnas.0608484103. Epub 2006 Nov 10. Proc Natl Acad Sci U S A. 2006. PMID: 17098863 Free PMC article. - The Role of Nucleoporin Elys in Nuclear Pore Complex Assembly and Regulation of Genome Architecture.
Shevelyov YY. Shevelyov YY. Int J Mol Sci. 2020 Dec 13;21(24):9475. doi: 10.3390/ijms21249475. Int J Mol Sci. 2020. PMID: 33322130 Free PMC article. Review.
Cited by
- Lumenal interactions in nuclear pore complex assembly and stability.
Yewdell WT, Colombi P, Makhnevych T, Lusk CP. Yewdell WT, et al. Mol Biol Cell. 2011 Apr 15;22(8):1375-88. doi: 10.1091/mbc.E10-06-0554. Epub 2011 Feb 23. Mol Biol Cell. 2011. PMID: 21346187 Free PMC article. - Drosophila ELYS regulates Dorsal dynamics during development.
Mehta SJK, Kumar V, Mishra RK. Mehta SJK, et al. J Biol Chem. 2020 Feb 21;295(8):2421-2437. doi: 10.1074/jbc.RA119.009451. Epub 2020 Jan 15. J Biol Chem. 2020. PMID: 31941789 Free PMC article. - Reorganization of the nuclear envelope during open mitosis.
Kutay U, Hetzer MW. Kutay U, et al. Curr Opin Cell Biol. 2008 Dec;20(6):669-77. doi: 10.1016/j.ceb.2008.09.010. Epub 2008 Nov 2. Curr Opin Cell Biol. 2008. PMID: 18938243 Free PMC article. Review. - Genetic Analyses of Elys Mutations in Drosophila Show Maternal-Effect Lethality and Interactions with Nucleoporin Genes.
Hirai K, Wang Z, Miura K, Hayashi T, Awasaki T, Wada M, Keira Y, Ishikawa HO, Sawamura K. Hirai K, et al. G3 (Bethesda). 2018 Jul 2;8(7):2421-2431. doi: 10.1534/g3.118.200361. G3 (Bethesda). 2018. PMID: 29773558 Free PMC article. - Orchestrating nuclear envelope disassembly and reassembly during mitosis.
Güttinger S, Laurell E, Kutay U. Güttinger S, et al. Nat Rev Mol Cell Biol. 2009 Mar;10(3):178-91. doi: 10.1038/nrm2641. Nat Rev Mol Cell Biol. 2009. PMID: 19234477 Review.
References
- Fernandez A.G., Piano F. MEL-28 is downstream of the Ran cycle and is required for nuclear-envelope function and chromatin maintenance. Curr. Biol. 2006;16:1757–1763. - PubMed
- Galy V., Askjaer P., Franz C., Lopez-Iglesias C., Mattaj I.W. MEL-28, a novel nuclear-envelope and kinetochore protein essential for zygotic nuclear-envelope assembly in C. elegans. Curr. Biol. 2006;16:1748–1756. - PubMed
- Kimura N., Takizawa M., Okita K., Natori O., Igarashi K., Ueno M., Nakashima K., Nobuhisa I., Taga T. Identification of a novel transcription factor, ELYS, expressed predominantly in mouse foetal haematopoietic tissues. Genes Cells. 2002;7:435–446. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous