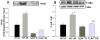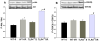Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion - PubMed (original) (raw)
Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion
Fang Hua et al. J Neuroimmunol. 2007 Oct.
Abstract
Toll-like receptors (TLRs) play a critical role in the induction of innate immune responses which have been implicated in neuronal death induced by global cerebral ischemia/reperfusion (GCI/R). The present study investigated the role and mechanisms-of-action of TLR4 signaling in ischemia-induced hippocampal neuronal death. Neuronal damage, activation of the TLR4 signaling pathway, expression of pro-inflammatory cytokines and activation of the PI3K/Akt signaling pathway in the hippocampal formation (HF) were assessed in wild type (WT) mice and TLR4 knockout (TLR4(-/-)) mice after GCI/R. GCI/R increased expression of TLR4 protein in the hippocampal formation (HF) and other brain structures in WT mice. Phosphorylation of the inhibitor of kappa B (p-IkappaB) as well as activation of nuclear factor kappa B (NFkappaB) increased in the HF of WT mice. In contrast, there were lower levels of p-IkappaB and NFkappaB binding activity in TLR4(-/-) mice subjected to GCI/R. Pro-inflammatory cytokine expression was also decreased, while phosphorylation of Akt and GSK3beta were increased in the HF of TLR4(-/-) mice after GCI/R. These changes correlated with decreased neuronal death/apoptosis in TLR4(-/-) mice following GCI/R. These data suggest that activation of TLR4 signaling contributes to ischemia-induced hippocampal neuronal death. In addition, these data suggest that modulation of TLR4 signaling may attenuate ischemic injury in hippocampal neurons.
Figures
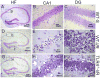
Figure 1. TLR4 deficient mice show decreased neuronal damage in the HF after GCI/R
Brain sections were stained with 0.1% cresyl violet. Viable neurons were defined as neurons in which a normal nucleus can be seen. Damaged neurons exhibit features including pyknosis, karyorhexis and shrunken cell bodies. Three days after GCI/R, neuronal damage was prominent in the CA1 and dentate gyrus (DG) of the HF in WT mice. Compared with WT mice, there was less neuronal damage in the HF of TLR4-/- mice. A, D and G: HF; B, E and H: CA1; C, F and I: dentate gyrus (DG). WT-S: wild type sham, WT-I/R: wild type subjected to GCI/R, TLR4-/- -I/R: TLR4-/- mice subjected to GCI/R.
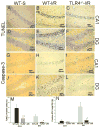
Figure 2. TLR4 deficiency results in decreased neuronal apoptosis in the HF in response to GCI/R
Neuronal apoptosis was confirmed by the methods of TUNEL staining (A to F) and IHC staining for cleaved caspase-3, a specific marker of apoptosis (G to L). The positive staining was shown in brown. Slides were counterstained with hematoxylin. Results show that GCI/R results in increased numbers of TUNEL positive cells in the HF (B and E) in WT mice. In TLR4-/--I/R mice, there were fewer TUNEL positive staining cells in the HF (C and F) compared with WT-I/R mice (M, # p < 0.05). GCI/R results in the expression of cleaved caspase-3 in the DG area of the HF in WT mice three days after GCI/R (K). There was less expression of cleaved caspase-3 in the DG in TLR4-/--I/R mice (L) compared with WT-I/R mice (N, # p < 0.01). In both WT and TLR4-/- mice, there was only light positive staining for cleaved caspase-3 in the CA1 of the HF three days after GCI/R (H and I). A, B, C, G, H and I: CA1; D, E, F, J, K and L: dentate gyrus (DG) of the HF. WT-S: wild type sham, WT-I/R: wild type subjected to GCI/R, TLR4-/- -I/R: TLR4-/- mice subjected to GCI/R.
Figure 3. TLR4 expression in WT mice is increased in brain tissue after GCI/R
Brain tissue sections were stained by IHC, TLR4 immunoreactivity was indicated by the presence of a brown color. Tissue sections were counterstained with hematoxylin. Results showed that there was no TLR4 expression observed in sham operated WT mice (WT-S) (A). Expression of TLR4 was observed in WT mice brain (B) after GCI/R in the caudate-putamen (CPu, D), CA1 (E), dentate gyrus (DG, F) and cortex (G). WT-S: wild type sham, WT-I/R: wild type subjected to GCI/R. No TLR4 immunoreactivity was observed in TLR4-/- mice subjected to GCI/R (C).
Figure 4. Decreased nuclear translocation of phospho-NFκB p65 in the HF of TLR4-/- mice in response to GCI/R
Phospho-NFκB p65 (p-NFκB p65) immunoreactivity was shown as a brown color by IHC staining. Tissue sections were counterstained with hematoxylin. There was no detectable p-NFκB p65 in sham operated mice (A and D). Nuclear translocation of p-NFκB p65 was increased in the HF of WT mice by GCI/R, shown in CA1 (B) and dentate gyrus (DG, E) (arrows). Less p-NFκB p65 was observed in the CA1 and DG of TLR4-/- mice after GCI/R compared with WT mice (B, C, E and F) (arrows). WT-S: wild type sham, WT-I/R: wild type subjected to GCI/R, TLR4-/- -I/R: TLR4-/- mice subjected to GCI/R.
Figure 5. Decreased NFκB DNA binding activity and increased phosphorylation of IκB in the HF of TLR4-/- mice after GCI/R
EMSA showed that GCI/R results in increased DNA binding activity of NFκB in the HF of WT mice six hours after GCI/R (A, #: compared with WT-S p< 0.05). The level of NFκB activity was less in TLR4-/- mice compared with WT mice six hours after GCI/R (A, *: compared with WT-I/R, p<0.05). Representative EMSA results are shown at the top. Western Blots showed that the level of phosphorylated IκB increased in the HF of WT mice 6 hours after GCI/R compared with WT control (B, #: compared with WT-S, p < 0.05). There was less p- IκB in the HF of TLR4-/- mice compared with WT mice 6 hours after GCI/R (B, *: compared with WT-I/R, p < 0.05). WT-S: wild type sham, WT-I/R: wild type subjected to GCI/R, TLR4-/--S: TLR4-/- sham, TLR4-/- -I/R: TLR4-/- mice subjected to GCI/R. Results are mean ± SE (n=3 in WT-S and TLR4-/--S groups; n=5 in WT-I/R and TLR4-/-I/R groups). Representative results of Western blots are shown at the top.
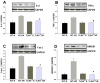
Figure 6. TLR4 dependent increase in IL-6, TNFα, Fas-L and HMGB1 expression in the HF in response to GCI/R
Western Blots showed that IL-6 (A), TNFα (B), Fas-L (C) and HMGB1 (D) levels were increased in the HF of WT mice 6 hours after GCI/R compared with WT sham control (#: compared with WT-S, p < 0.05). IL-6, TNFα, Fas-L and HMGB1 levels in the HF of TLR4-/- mice were not significantly changed after GCI/R and were lower than that in WT mice after GCI/R (*: compared with WT-I/R, p < 0.05). WT-S: wild type sham, WT-I/R: wild type subjected to GCI/R, TLR4-/--S: TLR4-/- sham, TLR4-/- -I/R: TLR4-/- mice subjected to GCI/R. Results are expressed as ratio of integrated density value (IDV) of individual cytokines vs. IDV of GAPDH, mean ± SE (n=3 in WT-S and TLR4-/--S groups; n=5 in WT-I/R and TLR4-/-I/R groups). Representative results of Western blots are shown at the top of each pane.
Figure 7. TLR4 deficiency results in increased brain phosphorylation of AKt and GSK3β in response GCI/R
Western Blots showed that the levels of phospho-Akt (p-Akt) (A) and phospho-GSK3β (p- GSK3β, B) in the HF of TLR4-/- mice were significantly higher than those in WT mice at 6 hours after GCI/R (*: compared with WT-I/R, p < 0.05; #: compared with WT-S, p < 0.05). WT-S: wild type sham, WT-I/R: wild type subjected to GCI/R, TLR4-/--S: TLR4-/- sham, TLR4-/- -I/R: TLR4-/- mice subjected to GCI/R. Results are expressed as ratio of integrated density value (IDV) of p-Akt vs. IDV of Akt1, and IDV of p- GSK3β vs. GSK3β, mean ± SE (n=3 in WT-S and TLR4-/--S groups; n=5 in WT-I/R and TLR4-/-I/R groups). Representative results of Western blots are shown at the top of each pane.
Similar articles
- Differential roles of TLR2 and TLR4 in acute focal cerebral ischemia/reperfusion injury in mice.
Hua F, Ma J, Ha T, Kelley JL, Kao RL, Schweitzer JB, Kalbfleisch JH, Williams DL, Li C. Hua F, et al. Brain Res. 2009 Mar 25;1262:100-8. doi: 10.1016/j.brainres.2009.01.018. Epub 2009 Jan 22. Brain Res. 2009. PMID: 19401158 Free PMC article. - Toll-like receptor 4-mediated myeloid differentiation factor 88-dependent signaling pathway is activated by cerebral ischemia-reperfusion in hippocampal CA1 region in mice.
Gao Y, Fang X, Sun H, Wang Y, Yao LJ, Li JP, Tong Y, Zhang B, Liu Y. Gao Y, et al. Biol Pharm Bull. 2009 Oct;32(10):1665-71. doi: 10.1248/bpb.32.1665. Biol Pharm Bull. 2009. PMID: 19801825 - Protection against myocardial ischemia/reperfusion injury in TLR4-deficient mice is mediated through a phosphoinositide 3-kinase-dependent mechanism.
Hua F, Ha T, Ma J, Li Y, Kelley J, Gao X, Browder IW, Kao RL, Williams DL, Li C. Hua F, et al. J Immunol. 2007 Jun 1;178(11):7317-24. doi: 10.4049/jimmunol.178.11.7317. J Immunol. 2007. PMID: 17513782 - Downregulation of Nogo-B ameliorates cerebral ischemia/reperfusion injury in mice through regulating microglia polarization via TLR4/NF-kappaB pathway.
Gong P, Jia HY, Li R, Ma Z, Si M, Qian C, Zhu FQ, Sheng-Yong L. Gong P, et al. Neurochem Int. 2023 Jul;167:105553. doi: 10.1016/j.neuint.2023.105553. Epub 2023 May 23. Neurochem Int. 2023. PMID: 37230196 Review. - NF-kappaB signaling in cerebral ischemia.
Ridder DA, Schwaninger M. Ridder DA, et al. Neuroscience. 2009 Feb 6;158(3):995-1006. doi: 10.1016/j.neuroscience.2008.07.007. Epub 2008 Jul 10. Neuroscience. 2009. PMID: 18675321 Review.
Cited by
- Increased expression of microRNA-146a decreases myocardial ischaemia/reperfusion injury.
Wang X, Ha T, Liu L, Zou J, Zhang X, Kalbfleisch J, Gao X, Williams D, Li C. Wang X, et al. Cardiovasc Res. 2013 Mar 1;97(3):432-42. doi: 10.1093/cvr/cvs356. Epub 2012 Dec 3. Cardiovasc Res. 2013. PMID: 23208587 Free PMC article. - A Review of the Biological Mechanisms of Dexmedetomidine for Postoperative Neurocognitive Disorders.
Yu S, Leng Y, Wang Y, Zhao G. Yu S, et al. Med Sci Monit. 2022 Oct 25;28:e937862. doi: 10.12659/MSM.937862. Med Sci Monit. 2022. PMID: 36281208 Free PMC article. Review. - Novel role for the innate immune receptor Toll-like receptor 4 (TLR4) in the regulation of the Wnt signaling pathway and photoreceptor apoptosis.
Yi H, Patel AK, Sodhi CP, Hackam DJ, Hackam AS. Yi H, et al. PLoS One. 2012;7(5):e36560. doi: 10.1371/journal.pone.0036560. Epub 2012 May 17. PLoS One. 2012. PMID: 22615780 Free PMC article. - The cytokine IL-27 reduces inflammation and protects photoreceptors in a mouse model of retinal degeneration.
Nortey A, Garces K, Carmy-Bennun T, Hackam AS. Nortey A, et al. J Neuroinflammation. 2022 Sep 5;19(1):216. doi: 10.1186/s12974-022-02576-x. J Neuroinflammation. 2022. PMID: 36064575 Free PMC article. - Silencing TLR4 using an ultrasound-targeted microbubble destruction-based shRNA system reduces ischemia-induced seizures in hyperglycemic rats.
Chen J, Huang F, Fang X, Li S, Liang Y. Chen J, et al. Open Life Sci. 2022 Dec 27;17(1):1689-1697. doi: 10.1515/biol-2022-0526. eCollection 2022. Open Life Sci. 2022. PMID: 36619717 Free PMC article.
References
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. - PubMed
- Barone F, Arvin B, White R, Miller A, Webb C, Willette R, Lysko P, Feuerstein G. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. - PubMed
- Barone F, Feuerstein G. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19:819–834. - PubMed
- Berti R, Williams A, Moffett J, Hale S, Velarde L, Elliott P, Yao C, Dave J, Tortella F. Quantitative real-time RT-PCR analysis of inflammatory gene expression associated with ischemia-reperfusion brain injury. J Cereb Blood Flow Metab. 2002;22:1068–1079. - PubMed
- Bottcher T, Von Mering M, Ebert S, Meyding-Lamade U, Kuhnt U, Gerber J, Nau R. Differential regulation of Toll-like receptor mRNAs in experimental murine central nervous system infections. Neurosci Lett. 2003;344:17–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous