Adaptive immune cells temper initial innate responses - PubMed (original) (raw)
. 2007 Oct;13(10):1248-52.
doi: 10.1038/nm1633. Epub 2007 Sep 23.
Affiliations
- PMID: 17891146
- PMCID: PMC2435248
- DOI: 10.1038/nm1633
Adaptive immune cells temper initial innate responses
Kwang Dong Kim et al. Nat Med. 2007 Oct.
Abstract
Toll-like receptors (TLRs) recognize conserved microbial structures called pathogen-associated molecular patterns. Signaling from TLRs leads to upregulation of co-stimulatory molecules for better priming of T cells and secretion of inflammatory cytokines by innate immune cells. Lymphocyte-deficient hosts often die of acute infection, presumably owing to their lack of an adaptive immune response to effectively clear pathogens. However, we show here that an unleashed innate immune response due to the absence of residential T cells can also be a direct cause of death. Viral infection or administration of poly(I:C), a ligand for TLR3, led to cytokine storm in T-cell- or lymphocyte-deficient mice in a fashion dependent on NK cells and tumor necrosis factor. We have further shown, through the depletion of CD4+ and CD8+ cells in wild-type mice and the transfer of T lymphocytes into Rag-1-deficient mice, respectively, that T cells are both necessary and sufficient to temper the early innate response. In addition to the effects of natural regulatory T cells, close contact of resting CD4+CD25-Foxp3- or CD8+ T cells with innate cells could also suppress the cytokine surge by various innate cells in an antigen-independent fashion. Therefore, adaptive immune cells have an unexpected role in tempering initial innate responses.
Figures
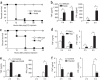
Figure 1. Comparison of mortality rates, liver virus titers and serum inflammatory cytokines of wild-type BALB/c mice and nude mice after MHV-A59 infection.
(a) Mortality curves of wild-type BALB/c (◊, n = 12) and nude mice (▪, n = 12) after infection with 4 × 105 PFU MHV-A59. (b) Liver virus titers were determined at day 2 (P = 0.051) and day 4 (P = 0.925) after infection (n = 11). (c) TNF and IFN-γ concentrations in serum were determined at day 2 after infection (pool of 14–16 mice in each group from various experiments). Horizontal bars indicate the median values. Statistical comparisons were significant. **P < 0.01, by _t_-test.
Figure 2. Wild-type mice are more resistant to poly(I:C) than nude mice and Rag-1 knockout mice, and T cells are essential for controlling proinflammatory cytokine production in vivo.
(a) Mortality curves of wild-type BALB/c (⋄, n = 7) and nude mice (▪, n = 10) after injection with 700 μg poly(I:C). (b) TNF and IFN-γ concentrations in serum were determined at 2 h and 6 h after poly(I:C) injection (n = 4–9). (c) Mortality curves of wild-type C57BL6 (⋄, n = 8) and Rag-1 knockout (▪, n = 16) mice after injection with 400 μg poly(I:C). (d) TNF and IFN-γ in serum of wild-type C57BL6 and Rag-1 knockout mice 2 h and 6 h after poly(I:C) injection. (e) TNF and IFN-γ in serum of T cell–depleted wild-type C57BL6 mice 2 h and 6 h after poly(I:C) injection. The results are representative of two experiments (n = 6 mice total, 3 per group). (f) Reduced cytokines in Rag-1 knockout mice reconstituted with lymphocytes. Rag-1 knockout mice (n = 4 per group) were adoptively transferred with lymphocytes for 2 d and then treated with poly(I:C); reconstituted mice produced significantly lower levels of cytokines than control mice. *P < 0.05, by _t_-test.
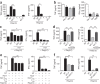
Figure 3. T cells inhibit proinflammatory cytokine production of splenocytes stimulated with poly(I:C) in vitro.
(a–c) 1 × 106 non-T cells were stimulated with 100 μg/ml poly(I:C) in the presence or absence of 1 × 106 pan-T (a), CD4+ T cells or CD8+ T cells (b), or OT-II transgenic CD4+ T cells or OT-I transgenic CD8+ T cells (c). (d) To prevent close contacts between non-T cells and T cells, non-T cells were cultured in the lower chamber of a Transwell system containing T cells in the upper chamber. (e,f) CD4+CD25+ (Treg) or CD4+CD25− (non-Treg) T cells from wild-type mouse with a ratio of non-T cell to T cell of 1:0.5 (e), or GFP− T cell (non-Treg) or GFP+ T cells (Treg) from FoxP3 knock-in mouse (f), were added to non-T cells. The ratio of non-T cells to GFP+ or GFP− T cell was 1:0.5. *P < 0.05, **P < 0.01, by _t_-test. The results are representative of at least two independent experiments for each panel (n = 6 per group).
Figure 4. TNFR-hIg can substantially improve survival rates among nude and Rag-1 knockout mice treated with poly(I:C), and NK cells are critical in the sudden death of Rag-1 knockout mice after poly(I:C) injection.
(a) T cells inhibited induction of TNF-producing CD11b+ or CD11c+ cells and IFN-γ–producing NK cells in vitro. (b) Decreased inflammatory cytokines from innate cells (CD11b+ cells, NK cells or both) after poly(I:C) in the presence of T cells. (c) Mortality curve of control hIg–treated (⋄, n = 10) and TNFR–hIg–treated nude mice (▪, n = 5) after injection of 700 μg poly(I:C). (d) Mortality curve of Rag-1 knockout mice (hIg: ⋄, n = 5 and TNFR-hIg: ▪, n = 5) after poly(I:C) injection. (e) Decreased inflammatory cytokines from non-T cells after poly(I:C) stimulation and addition of soluble TNFR-hIg. (f) Mortality curve of Rag-1 knockout (⋄, n = 8) and NK-depleted Rag-1 knockout (▪, n = 8) mice. (g) Levels of proinflammatory cytokines in serum from Rag-1 knockout and NK-depleted (NK−) Rag-1 knockout mice were determined at 2 and 6 h after poly(I:C) injection. *P < 0.05, **P < 0.01, by _t_-test.
Comment in
- Not so fast: adaptive suppression of innate immunity.
Palm NW, Medzhitov R. Palm NW, et al. Nat Med. 2007 Oct;13(10):1142-4. doi: 10.1038/nm1007-1142b. Nat Med. 2007. PMID: 17917657 Free PMC article. - Hyperinflammation, T cells, and endotoxemia.
Inoue M, Shinohara ML. Inoue M, et al. Oncotarget. 2015 Sep 15;6(27):23040-1. doi: 10.18632/oncotarget.5236. Oncotarget. 2015. PMID: 26309079 Free PMC article. No abstract available.
Similar articles
- CD4+CD25+ regulatory T cells control innate immune reactivity after injury.
Murphy TJ, Ni Choileain N, Zang Y, Mannick JA, Lederer JA. Murphy TJ, et al. J Immunol. 2005 Mar 1;174(5):2957-63. doi: 10.4049/jimmunol.174.5.2957. J Immunol. 2005. PMID: 15728508 - Insufficient Innate Immunity Contributes to the Susceptibility of the Castaneous Mouse to Orthopoxvirus Infection.
Earl PL, Americo JL, Moss B. Earl PL, et al. J Virol. 2017 Sep 12;91(19):e01042-17. doi: 10.1128/JVI.01042-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28747505 Free PMC article. - Natural killer cells prevent CD28-mediated Foxp3 transcription in CD4+CD25- T lymphocytes.
Brillard E, Pallandre JR, Chalmers D, Ryffel B, Radlovic A, Seilles E, Rohrlich PS, Pivot X, Tiberghien P, Saas P, Borg C. Brillard E, et al. Exp Hematol. 2007 Mar;35(3):416-25. doi: 10.1016/j.exphem.2006.12.004. Exp Hematol. 2007. PMID: 17309822 - Emerging concepts in autoimmune encephalomyelitis beyond the CD4/T(H)1 paradigm.
Batoulis H, Addicks K, Kuerten S. Batoulis H, et al. Ann Anat. 2010 Aug 20;192(4):179-93. doi: 10.1016/j.aanat.2010.06.006. Epub 2010 Jul 15. Ann Anat. 2010. PMID: 20692821 Review. - Licensing of killer dendritic cells in mouse and humans: functional similarities between IKDC and human blood γδ T-lymphocytes.
Anderson J, Gustafsson K, Himoudi N. Anderson J, et al. J Immunotoxicol. 2012 Jul-Sep;9(3):259-66. doi: 10.3109/1547691X.2012.685528. Epub 2012 May 27. J Immunotoxicol. 2012. PMID: 22632132 Review.
Cited by
- Antiviral effects of small interfering RNA simultaneously inducing RNA interference and type 1 interferon in coxsackievirus myocarditis.
Ahn J, Ko A, Jun EJ, Won M, Kim YK, Ju ES, Jeon ES, Lee H. Ahn J, et al. Antimicrob Agents Chemother. 2012 Jul;56(7):3516-23. doi: 10.1128/AAC.06050-12. Epub 2012 Apr 16. Antimicrob Agents Chemother. 2012. PMID: 22508300 Free PMC article. - Lessons for COVID-19 Immunity from Other Coronavirus Infections.
Sariol A, Perlman S. Sariol A, et al. Immunity. 2020 Aug 18;53(2):248-263. doi: 10.1016/j.immuni.2020.07.005. Epub 2020 Jul 14. Immunity. 2020. PMID: 32717182 Free PMC article. Review. - NKG2D blockade inhibits poly(I:C)-triggered fetal loss in wild type but not in IL-10-/- mice.
Thaxton JE, Nevers T, Lippe EO, Blois SM, Saito S, Sharma S. Thaxton JE, et al. J Immunol. 2013 Apr 1;190(7):3639-47. doi: 10.4049/jimmunol.1203488. Epub 2013 Mar 1. J Immunol. 2013. PMID: 23455498 Free PMC article. - Extrapulmonary manifestations of COVID-19.
Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. Gupta A, et al. Nat Med. 2020 Jul;26(7):1017-1032. doi: 10.1038/s41591-020-0968-3. Epub 2020 Jul 10. Nat Med. 2020. PMID: 32651579 Review. - Aging-induced fragility of the immune system.
Jones E, Sheng J, Carlson J, Wang S. Jones E, et al. J Theor Biol. 2021 Feb 7;510:110473. doi: 10.1016/j.jtbi.2020.110473. Epub 2020 Sep 14. J Theor Biol. 2021. PMID: 32941914 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK058897-05/DK/NIDDK NIH HHS/United States
- DK58891/DK/NIDDK NIH HHS/United States
- 5 T32 GM07281/GM/NIGMS NIH HHS/United States
- R01 DK058897-06/DK/NIDDK NIH HHS/United States
- R01 AI062026-07/AI/NIAID NIH HHS/United States
- AI062026/AI/NIAID NIH HHS/United States
- T32 GM007281/GM/NIGMS NIH HHS/United States
- CA115540/CA/NCI NIH HHS/United States
- R01 AI062026-06/AI/NIAID NIH HHS/United States
- R01 DK058897-07/DK/NIDDK NIH HHS/United States
- R01 AI062026-08/AI/NIAID NIH HHS/United States
- R01 AI062026/AI/NIAID NIH HHS/United States
- R01 CA115540-03/CA/NCI NIH HHS/United States
- R01 CA115540/CA/NCI NIH HHS/United States
- R01 DK058897/DK/NIDDK NIH HHS/United States
- R01 DK058891/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials