Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens - PubMed (original) (raw)
Comparative Study
Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens
Huiyuan Zheng et al. J Neurosci. 2007.
Abstract
The overriding of satiety and homeostatic control mechanisms by cognitive, rewarding, and emotional aspects of palatable foods may contribute to the evolving obesity crisis, but little is known about neural pathways and mechanisms responsible for crosstalk between the "cognitive" and "metabolic" brain in the control of appetite. Here we show that neural connections between the nucleus accumbens and hypothalamus might be part of this link. Using the well known model of selective stimulation of high-fat intake induced by intra-accumbens injection of the mu-opioid receptor agonist D-Ala2-N-Me-Phe4-gly5-ol-enkephalin (DAMGO), we demonstrate that orexin signaling in the ventral tegmental area is important for this reward-driven appetite to override metabolic repletion signals in presatiated rats. We further show that accumbens DAMGO in the absence of food selectively increases the proportion of orexin neurons expressing c-Fos in parts of the perifornical hypothalamus and that neural projections originating in DAMGO-responsive sites of the nucleus accumbens make close anatomical contacts with hypothalamic orexin neurons. These findings suggest that direct accumbens-hypothalamic projections can stimulate hypothalamic orexin neurons, which in turn through orexin-1 receptor signaling in the ventral tegmental area and possibly other sites interfaces with the motivational and motor systems to increase intake of palatable food.
Figures
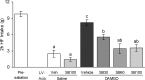
Figure 1.
Orexin-1 receptor antagonist administration into the lateral ventricle attenuates high-fat intake induced by intra-accumbens injection of DAMGO in presatiated rats. Rats maintained on chow diet in were given 1 h access to high-fat diet. Immediately thereafter, they received either vehicle (Veh) or SB334867 (SB; 30, 60, or 100 nmol) injections into the right lateral ventricle (LV) and either saline or DAMGO (250 ng) injections into the nucleus accumbens (Acb). Intake of high-fat (HF) diet was measured for 2 h after accumbens injections. Bars that do not share the same letter are significantly different from each other (based on ANOVA, followed by Bonferroni's adjusted multiple comparisons test, p < 0.05).
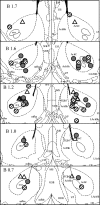
Figure 2.
DAMGO injection sites in the nucleus accumbens. Location of injection cannula tips in the nucleus accumbens of rats used for experiments with lateral ventricle administration (open triangles) and for experiments with intra-VTA administration (in VTA, striped circles; outside VTA, gray circles) of the orexin receptor antagonist SB334867. Injection sites are superimposed on images from the Paxinas and Watson (1997) stereotaxic atlas.
Figure 3.
Orexin-1 receptor antagonist administration into the VTA blocks high-fat intake induced by accumbens administration of DAMGO. A, Vehicle (Veh) or the orexin receptor antagonist SB334867 (SB) (15 nmol/side) was injected into the VTA and saline or DAMGO (250 ng) into the nucleus accumbens after overnight access to high-fat (HF) chow for presatiation. The robust DAMGO-induced feeding response over saline baseline was almost completely abolished by VTA pretreatment with the orexin receptor antagonist (NS). In animals with either one or both of the bilateral cannula tips not within the VTA, the orexin receptor antagonist was unable to block DAMGO-induced high-fat feeding. Bars that do not share the same letter are significantly (p < 0.05) different from each other, based on ANOVA. B, Verification of orexin receptor antagonist injection sites aimed at the VTA. Striped circles depict animals with both sites within the VTA (n = 11), gray circles depict animals with one or both sites outside the VTA (n = 6), and diamond-filled circles depict animals with unilateral injections (n = 2). Injection sites are superimposed on images from the Paxinos and Watson (1997) stereotaxic atlas.
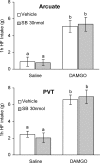
Figure 4.
Orexin-1 receptor antagonist administration into the arcuate nucleus or PVT does not affect high-fat (HF) intake induced by accumbens DAMGO. Vehicle or the orexin receptor antagonist SB334867 (SB) (15 nmol/side) was injected into the arcuate nucleus of the hypothalamus (top) or the PVT (bottom) and saline or DAMGO (250 ng) into the nucleus accumbens immediately after the 1 h presatiation period with high-fat chow. In contrast to the VTA, orexin receptor antagonist administration into the arcuate nucleus and PVT did not change DAMGO-induced high-fat intake. Bars that do not share the same letter are significantly (p < 0.01) different from each other, based on ANOVA.
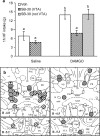
Figure 5.
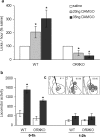
Orexin-deficient mice do not increase high-fat intake after intra-accumbens injection of DAMGO. a, DAMGO (20 or 35 ng) administered to the nucleus accumbens significantly increases intake of 20% corn oil in wild-type (WT) (n = 5) but not in orexin knock-out (ORXKO) (n = 6) mice during the second hour of access. * indicates significantly different compared with saline control. b, DAMGO (35 ng) administered unilaterally to the nucleus accumbens increases locomotor activity in both wild-type and orexin-deficient mice during the first but not during the second hour of access to corn oil. c, Injection sites in wild-type (gray circles) and orexin knock-out (striped circles) mice. Injection sites are superimposed on images from the Paxinos and Watson (1997) stereotaxic atlas.
Figure 6.
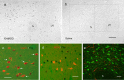
DAMGO induces c-Fos in hypothalamic orexin neurons, and DAMGO-responsive sites project to the hypothalamic orexin field in rats. a, b, DAMGO (250 ng) but not saline administered to the nucleus accumbens increases c-Fos expression in the perifornical hypothalamus. Rectangular boxes indicate the areas selected for quantitative analysis in Figure 4. c, DAMGO induces c-Fos expression (black nuclei) in many orexin-immunoreactive neurons (red cytoplasm). Examples of double-labeled neurons are indicated by arrows. d, e, Retrograde tracing of axonal projections from DAMGO-responsive nucleus accumbens site to the perifornical hypothalamus. Lower- and higher-magnification images of BDA-labeled axon profiles (red) in close anatomical apposition to orexin-IR neurons (green) in perifornical area of hypothalamus. fx, Fornix. Scale bars: a, b, 200 μm; c–e, 50 μm.
Figure 7.
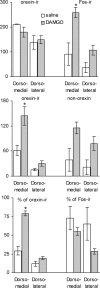
DAMGO significantly increases the percentage of Fos-expressing orexin neurons in selective areas of the hypothalamus. Top, Numbers of neurons expressing c-Fos after intra-accumbens DAMGO or saline injection and orexin-immunoreactive neurons in two areas of the hypothalamus (see Fig. 6). Middle, Numbers of Fos-IR neurons expressing orexin immunoreactivity (double-labeled) and non-orexin neurons expressing Fos immunoreactivity. Bottom, Double-labeled neurons expressed as percentage of orexin-IR and Fos-IR neurons. Note that the number and percentage of orexin-IR neurons expressing DAMGO-induced c-Fos (double-labeled neurons) is significantly higher compared with saline (*p < 0.05) only in the area dorsomedial to the fornix.
Similar articles
- Neural activation patterns underlying basolateral amygdala influence on intra-accumbens opioid-driven consummatory versus appetitive high-fat feeding behaviors in the rat.
Parker KE, McCabe MP, Johns HW, Lund DK, Odu F, Sharma R, Thakkar MM, Cornelison DD, Will MJ. Parker KE, et al. Behav Neurosci. 2015 Dec;129(6):812-21. doi: 10.1037/bne0000095. Epub 2015 Oct 26. Behav Neurosci. 2015. PMID: 26501175 Free PMC article. - Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network.
Will MJ, Franzblau EB, Kelley AE. Will MJ, et al. J Neurosci. 2003 Apr 1;23(7):2882-8. doi: 10.1523/JNEUROSCI.23-07-02882.2003. J Neurosci. 2003. PMID: 12684475 Free PMC article. - Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction.
Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Aston-Jones G, et al. Brain Res. 2010 Feb 16;1314:74-90. doi: 10.1016/j.brainres.2009.09.106. Epub 2009 Oct 6. Brain Res. 2010. PMID: 19815001 Free PMC article. Review. - Role of orexin/hypocretin in dependence and addiction.
Sharf R, Sarhan M, Dileone RJ. Sharf R, et al. Brain Res. 2010 Feb 16;1314:130-8. doi: 10.1016/j.brainres.2009.08.028. Epub 2009 Aug 20. Brain Res. 2010. PMID: 19699189 Free PMC article. Review.
Cited by
- Modulation of opioid-induced feeding behavior by endogenous nitric oxide in neonatal layer-type chicks.
Alimohammadi S, Zendehdel M, Babapour V. Alimohammadi S, et al. Vet Res Commun. 2015 Jun;39(2):105-13. doi: 10.1007/s11259-015-9631-8. Epub 2015 Feb 13. Vet Res Commun. 2015. PMID: 25677536 - Nociceptin induces hypophagia in the perifornical and lateral hypothalamic area.
Parsons MP, Burt J, Cranford A, Alberto C, Zipperlen K, Hirasawa M. Parsons MP, et al. PLoS One. 2012;7(9):e45350. doi: 10.1371/journal.pone.0045350. Epub 2012 Sep 17. PLoS One. 2012. PMID: 23028954 Free PMC article. - Multiple roles for orexin/hypocretin in addiction.
Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Mahler SV, et al. Prog Brain Res. 2012;198:79-121. doi: 10.1016/B978-0-444-59489-1.00007-0. Prog Brain Res. 2012. PMID: 22813971 Free PMC article. Review. - Glucose utilization rates regulate intake levels of artificial sweeteners.
Tellez LA, Ren X, Han W, Medina S, Ferreira JG, Yeckel CW, de Araujo IE. Tellez LA, et al. J Physiol. 2013 Nov 15;591(22):5727-44. doi: 10.1113/jphysiol.2013.263103. Epub 2013 Sep 23. J Physiol. 2013. PMID: 24060992 Free PMC article. - Delta FosB-mediated alterations in dopamine signaling are normalized by a palatable high-fat diet.
Teegarden SL, Nestler EJ, Bale TL. Teegarden SL, et al. Biol Psychiatry. 2008 Dec 1;64(11):941-50. doi: 10.1016/j.biopsych.2008.06.007. Epub 2008 Jul 26. Biol Psychiatry. 2008. PMID: 18657800 Free PMC article.
References
- Backberg M, Hervieu G, Wilson S, Meister B. Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. Eur J Neurosci. 2002;15:315–328. - PubMed
- Balcita-Pedicino JJ, Sesack SR. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. J Comp Neurol. 2007;503:668–684. - PubMed
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. - PubMed
- Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. - PubMed
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK071082/DK/NIDDK NIH HHS/United States
- P30 DK072476/DK/NIDDK NIH HHS/United States
- DK071082/DK/NIDDK NIH HHS/United States
- R01 DK047348/DK/NIDDK NIH HHS/United States
- DK47348/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials