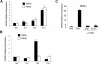Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway - PubMed (original) (raw)
Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway
Kevin M Elias et al. Blood. 2008.
Abstract
CD4(+) helper T (Th) cells play a crucial role in the delicate balance between host defense and autoimmune disease. Two important populations of helper T cells are the proinflammatory, interleukin-17 (IL-17)-producing (Th17) cells and the anti-inflammatory forkhead box P3-positive (FoxP3(+)) T regulatory (Treg) cells. Here we show that all-trans retinoic acid (ATRA) and other agonists of the retinoic acid receptor alpha (RARalpha) inhibit the formation of Th17 cells and promote FoxP3 expression. Conversely, inhibition of retinoic acid signaling constrains transforming growth factor beta (TGF-beta1) induction of FoxP3. The effect of ATRA is mediated independently of IL-2, signal transducer and activator of transcription 5 (Stat5) and Stat3, representing a novel mechanism for the induction of FoxP3 in CD4 T cells. As previous studies have shown that vitamin A derivatives are protective in animal models of autoimmune disease, the current data suggest a previously unrecognized role for RARalpha in the regulation of CD4(+) T-cell differentiation and provide a mechanism for the anti-inflammatory effects of retinoic acid.
Figures
Figure 1
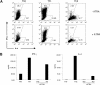
ATRA inhibits Th1 and Th2 polarization. Polyclonal CD4+CD62L+ cells isolated by MACS from C57Bl6J mice were polarized for 5 days under neutral, Th1 (IL-12 and anti–IL-4), or Th2 (IL-4 and anti–IFN-γ) favoring culture conditions with or without 1 μM ATRA. (A) Cytokine production at day 5 by intracellular staining. Numbers on plots are percentages of total cells. (B) Cells were washed thoroughly after 5 days in culture, then resuspended at 0.5 × 106 cells/mL and restimulated overnight with plate-bound anti-CD3 and anti-CD28 in media without additional cytokines. Supernatants were collected and measured by ELISA; error bars represent standard deviations (n = 3). These experiments are representative of 3 independent experiments.
Figure 2
ATRA inhibits Th17 polarization and promotes FoxP3 expression. (A) CD4+ T cells isolated by MACS beads from OTII TCR transgenic mice were incubated with isolated CD11c+ cells pulsed with 1 μg OVA peptide and cultured for 3 days under neutral or Th17-favoring (IL-6, TGF-β1, anti–IFN-γ, and anti–IL-4) conditions with or without 1 μM ATRA. The figure depicts intracellular staining for IL-17 production and FoxP3 expression, and it is representative of 2 independent experiments. Numbers on plots are percentages of total cells. (B,C) Polyclonal CD4+CD62L+CD25−CD44− cells isolated by flow cytometry were stimulated for 3 days with anti-CD3 and anti-CD28 under neutral or Th17-favoring conditions with or without ATRA. (B) Average IL-17 concentration in supernatants from 3 independent experiments as measured by ELISA. The P value was determined by a 2-tailed paired t test; a single asterisk denotes significance (P < .05). (C) FoxP3 expression normalized to β-actin levels and relative to Th-neutral conditions without ATRA; error bars represent standard deviation (n = 3); the data are representative of 2 independent experiments.
Figure 3
Th17 cells express high levels of RARα, RARγ, and RORγt mRNA. (A,B) CD4+CD62L+ cells isolated by MACS were polyclonally stimulated for 3 days under Th-neutral, Th1, Th2, or Th17 conditions. Relative expression of RAR and RXR receptors was analyzed by quantitative RT-PCR. Expression is normalized to β-actin levels under Th-neutral conditions, and error bars represent standard deviation (n = 3). Detected RAR and RXR receptors are shown (RAR-β and RXR-γ were not present). (C) Naive CD4+ T cells were polyclonally stimulated for 3 days under Th-neutral or Th17 conditions in the presence or absence of 1 μM ATRA. Relative expression of RORγt mRNA was analyzed by quantitative RT-PCR. Expression is normalized to β-actin levels and relative to Th-neutral conditions; error bars represent standard deviation (n = 3). The inhibition of RORγt mRNA was confirmed in 3 independent experiments.
Figure 4
RARα is necessary for TGF-β1 induction of FoxP3. (A) Naive CD4+ cells were polyclonally stimulated for 3 days under Th17 conditions or TGF-β1 alone for 3 days with TTNBP (pan-RAR agonist) or LE540 (pan-RAR antagonist). IL-17 and FoxP3 expression were measured on fixed cells by intracellular staining. Numbers on plots are percentages of total cells. (B) Naive CD4+ T cells were polyclonally stimulated for 3 days with TGF-β1 and 1 μM ATRA, 1 μM A7980 (selective RARγ agonist), or 1 μM AM580 (selective RARα agonist). FoxP3 expression was measured on fixed cells by intracellular staining. Data are representative of 2 independent experiments. Numbers on graphs are percentages of cells that are FoxP3.+
Figure 5
ATRA enhances FoxP3 expression independent of Stat3. Peripheral CD4+ cells from Stat3 fl/fl CD4-Cre mice or WT controls were polyclonally stimulated for 3 days under Th-neutral conditions, Th17 conditions, or with TGF-β1 alone in the presence or absence of 1 μM ATRA. IL-17 and FoxP3 expression was measured on fixed cells by intracellular staining. Data are representative of 2 independent experiments. Numbers on plots are percentages of total cells.
Figure 6
ATRA enhances FoxP3 expression independent of Stat5. CD8-depleted thymocytes from Stat5 fl/fl CD4-Cre mice were polyclonally stimulated for 3 days with media alone, IL-6, IL-2, and TGF-β1, or IL-2 and TGFβ-1 in the presence or absence of 1 μM ATRA. IL-17 and FoxP3 expression were measured on CD4+ gated fixed cells by intracellular staining. Data are representative of 2 independent experiments. Numbers on plots are percentages of total cells.
Figure 7
ATRA enhances FoxP3 expression independent of IL-2. (A) Peripheral CD4 cells from Rag2−/− transgenic (OTII) mice were polyclonally stimulated for 3 days under Th17 conditions or TGF-β1 alone, in the presence of either IL-2 or anti–IL-2. All stimulations were performed with (lower panels) or without (upper panels) 1μM ATRA. IL-17 and FoxP3 expression were measured on fixed cells by intracellular staining. Data are representative of 2 independent experiments. Numbers on plots are percentages of total cells. (B) CD4+CD25− MACS-purified FoxP3-GFP cells were polyclonally stimulated for 3 days in the presence of TGF-β1, ATRA, and anti–IL-2. FoxP3-positive cells were isolated by flow cytometry and used to inhibit CD4+CD25− cells sorted by flow cytometry and stimulated with soluble anti-CD3 in the presence of irradiated Thy1.1− cells. Proliferation was measured by 3H-thymidine uptake and was compared with CD4+CD25+ (natural Treg) cells purified by flow cytometry. Error bars denote the standard error of the mean (n = 3), and significance was determined by an unpaired t test, and data are representative of 2 independent experiments.
Similar articles
- Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation.
Schambach F, Schupp M, Lazar MA, Reiner SL. Schambach F, et al. Eur J Immunol. 2007 Sep;37(9):2396-9. doi: 10.1002/eji.200737621. Eur J Immunol. 2007. PMID: 17694576 - IL-6-gp130-STAT3 in T cells directs the development of IL-17+ Th with a minimum effect on that of Treg in the steady state.
Nishihara M, Ogura H, Ueda N, Tsuruoka M, Kitabayashi C, Tsuji F, Aono H, Ishihara K, Huseby E, Betz UA, Murakami M, Hirano T. Nishihara M, et al. Int Immunol. 2007 Jun;19(6):695-702. doi: 10.1093/intimm/dxm045. Epub 2007 May 9. Int Immunol. 2007. PMID: 17493959 - Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression.
Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK. Xiao S, et al. J Immunol. 2008 Aug 15;181(4):2277-84. doi: 10.4049/jimmunol.181.4.2277. J Immunol. 2008. PMID: 18684916 Free PMC article. - The T helper 17-regulatory T cell axis in transplant rejection and tolerance.
Mitchell P, Afzali B, Lombardi G, Lechler RI. Mitchell P, et al. Curr Opin Organ Transplant. 2009 Aug;14(4):326-31. doi: 10.1097/MOT.0b013e32832ce88e. Curr Opin Organ Transplant. 2009. PMID: 19448538 Review. - Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation.
Chen Z, Laurence A, O'Shea JJ. Chen Z, et al. Semin Immunol. 2007 Dec;19(6):400-8. doi: 10.1016/j.smim.2007.10.015. Epub 2007 Dec 31. Semin Immunol. 2007. PMID: 18166487 Free PMC article. Review.
Cited by
- Leukocyte homing, fate, and function are controlled by retinoic acid.
Guo Y, Brown C, Ortiz C, Noelle RJ. Guo Y, et al. Physiol Rev. 2015 Jan;95(1):125-48. doi: 10.1152/physrev.00032.2013. Physiol Rev. 2015. PMID: 25540140 Free PMC article. Review. - Paradoxical effects of all-trans-retinoic acid on lupus-like disease in the MRL/lpr mouse model.
Liao X, Ren J, Wei CH, Ross AC, Cecere TE, Jortner BS, Ahmed SA, Luo XM. Liao X, et al. PLoS One. 2015 Mar 16;10(3):e0118176. doi: 10.1371/journal.pone.0118176. eCollection 2015. PLoS One. 2015. PMID: 25775135 Free PMC article. - Equilibrium of Treg/Th17 cells of peripheral blood in syphilitic patients with sero-resistance.
Zhao J, Ma J, Zhang X, Li Q, Yang X. Zhao J, et al. Exp Ther Med. 2016 Jun;11(6):2300-2304. doi: 10.3892/etm.2016.3178. Epub 2016 Mar 23. Exp Ther Med. 2016. PMID: 27284313 Free PMC article. - All-trans retinoic acid attenuates airway inflammation by inhibiting Th2 and Th17 response in experimental allergic asthma.
Wu J, Zhang Y, Liu Q, Zhong W, Xia Z. Wu J, et al. BMC Immunol. 2013 Jun 22;14:28. doi: 10.1186/1471-2172-14-28. BMC Immunol. 2013. PMID: 23800145 Free PMC article. - Intestinal permeability disturbances: causes, diseases and therapy.
Macura B, Kiecka A, Szczepanik M. Macura B, et al. Clin Exp Med. 2024 Sep 28;24(1):232. doi: 10.1007/s10238-024-01496-9. Clin Exp Med. 2024. PMID: 39340718 Free PMC article. Review.
References
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. - PubMed
- Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. - PubMed
- Zhu J, Yamane H, Cote-Sierra J, Guo L, Paul WE. GATA-3 promotes Th2 responses through three different mechanisms: induction of Th2 cytokine production, selective growth of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res. 2006;16:3–10. - PubMed
- Ochs HD, Ziegler SF, Torgerson TR. FOXP3 acts as a rheostat of the immune response. Immunol Rev. 2005;203:156–164. - PubMed
- Wu Y, Borde M, Heissmeyer V, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous