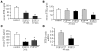The parathyroid is a target organ for FGF23 in rats - PubMed (original) (raw)
The parathyroid is a target organ for FGF23 in rats
Iddo Z Ben-Dov et al. J Clin Invest. 2007 Dec.
Abstract
Phosphate homeostasis is maintained by a counterbalance between efflux from the kidney and influx from intestine and bone. FGF23 is a bone-derived phosphaturic hormone that acts on the kidney to increase phosphate excretion and suppress biosynthesis of vitamin D. FGF23 signals with highest efficacy through several FGF receptors (FGFRs) bound by the transmembrane protein Klotho as a coreceptor. Since most tissues express FGFR, expression of Klotho determines FGF23 target organs. Here we identify the parathyroid as a target organ for FGF23 in rats. We show that the parathyroid gland expressed Klotho and 2 FGFRs. The administration of recombinant FGF23 led to an increase in parathyroid Klotho levels. In addition, FGF23 activated the MAPK pathway in the parathyroid through ERK1/2 phosphorylation and increased early growth response 1 mRNA levels. Using both rats and in vitro rat parathyroid cultures, we show that FGF23 suppressed both parathyroid hormone (PTH) secretion and PTH gene expression. The FGF23-induced decrease in PTH secretion was prevented by a MAPK inhibitor. These data indicate that FGF23 acts directly on the parathyroid through the MAPK pathway to decrease serum PTH. This bone-parathyroid endocrine axis adds a new dimension to the understanding of mineral homeostasis.
Figures
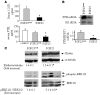
Figure 1. Klotho is expressed in the rat parathyroid.
(A) Immunoblot of microdissected parathyroid (PT), thyroid, and kidney using antibodies for Klotho and α-tubulin as loading control. (B) Real-time RT-PCR for membrane Klotho mRNA corrected for HPRT1 mRNA from microdissected parathyroid, thyroid, kidney, duodenum, liver, and spleen. Results are expressed as a percentage of expression in total kidney. Klotho mRNA expression is restricted to the parathyroid and kidney. (C) Immunohistochemistry with antibody for Klotho, FGFR1, and FGFR3 in thyroparathyroid tissue, showing specific expression of Klotho protein in the parathyroid and not in the surrounding thyroid (T) tissue. FGFR1 and -3 are also expressed in the parathyroid. A negative control without the primary antibody showed no staining (data not shown). Original magnification, ×100.
Figure 2. FGF23R176Q/R179Q decreases serum 1,25(OH)2–vitamin D, serum PTH, and PTH mRNA levels; increases parathyroid Klotho protein; and induces ERK phosphorylation in long-term experiments.
FGF23R176Q/R179Q (FGF23), FGF23core, or HEPES (control) were given daily for 5 days. (A) Serum 1,25(OH)2–vitamin D and PTH levels were decreased by FGF23R176Q/R179Q compared with FGF23core or HEPES. The results are expressed as mean ± SEM (n = 5). *P < 0.05 compared with control or FGF23core. (B) FGF23R176Q/R179Q decreased PTH mRNA levels as measured by northern blot (a pool from 3 rats in each lane) and by qRT-PCR. The results are expressed as mean ± SEM (n = 5). *P < 0.05. (C) Immunoblots for Klotho, phospho-ERK1/2, total ERK1/2, and α-tubulin of microdissected parathyroids from rats given FGF23R176Q/R179Q or FGF23core as described above. Each lane represents parathyroid extracts from a single rat. Results from 2 rats are shown for FGF23core and 3 rats for FGF23R176Q/R179Q. All samples were run on the same blot.
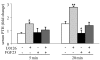
Figure 3. FGF23 decreases serum PTH and PTH mRNA levels in vivo in short-term experiments.
FGF23R176Q/R179Q, FGF23core, FGF23 wild-type (FGF23wt), or HEPES were injected i.v. or i.p. into 4 rats per group. At the indicated times, serum PTH levels were measured and parathyroids extracted for RNA analysis. (A) Serum PTH levels at 10 and 30 minutes after i.v. FGF23R176Q/R179Q or HEPES administration. (B) Serum PTH levels from untreated rats (control) and rats 20 or 40 minutes after i.p. FGF23R176Q/R179Q or FGF23core treatment. (C) Serum PTH at 24 hours after i.p. HEPES, FGF23R176Q/R179Q, or FGF23wt administration. (D) qRT-PCR analysis for PTH mRNA of RNA extracted from microdissected parathyroids 40 minutes after FGF23R176Q/R179Q or FGF23core treatment. FGF23R176Q/R179Q and FGF23wt administration decreased serum PTH and PTH mRNA levels. *P < 0.05 compared with FGF23core and (where applicable) controls.
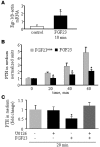
Figure 4. Local exposure to the MAPK inhibitor U0126 prevents i.v. FGF23R176Q/R179Q from decreasing serum PTH levels.
Rat parathyroid glands were exposed and submerged in PBS with or without ERK1/2 inhibitor (U0126, 1 μM), immediately followed by i.v. injection of FGF23R176Q/R179Q (+; 5 ng/rat) or HEPES carrier (–). Serum PTH levels at 5 and 20 minutes were normalized to pretreatment baseline values (n = 4 in each group). *P < 0.05; **P < 0.005 compared to the control group at each time point.
Figure 5. FGF23 decreases PTH secretion in vitro in isolated rat parathyroid glands.
(A) Egr-1 mRNA levels. Pairs of parathyroid glands were preincubated for 1 hour and then incubated without or with FGF23R176Q/R179Q for 10 minutes. Glands were extracted and analyzed by qRT-PCR for Egr-1 and β-actin. *P < 0.05. (B) Parathyroid glands from each rat were preincubated in medium for 60 minutes (time 0) and then transferred to medium containing either FGF23core or FGF23R176Q/R179Q (n = 13 rats per group). Media were sampled for PTH at the indicated time points. The accumulated PTH level of each parathyroid gland pair was measured and normalized to PTH in the medium of the glands at time 0. PTH secretion was inhibited by FGF23R176Q/R179Q compared with FGF23core at 20, 40, and 60 minutes. *P < 0.05. (C) As described above, pairs of parathyroid glands were incubated in control medium or in medium with U0126, FGF23R176Q/R179Q alone, or FGF23R176Q/R179Q with U0126 (n = 7, 6, 7, and 4 rats, respectively). Medium was sampled for PTH at 20 minutes and is presented as fold change in PTH levels compared with time 0. *P < 0.05 for the comparison with control and with the U0126 + FGF23 treatment group.
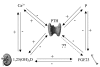
Figure 6. Endocrinological feedback loops in mineral homeostasis.
There are endocrinologic feedback loops between PTH, Ca2+, phosphorus, and 1,25(OH)2–vitamin D. Phosphorus and 1,25(OH)2–vitamin D levels are both trophic to FGF23, which in turn decreases phosphorus and 1,25(OH)2–vitamin D levels. We now show that FGF23 decreases serum PTH and PTH gene expression by activating its cognate FGFRs in the parathyroid in a Klotho-dependent fashion.
Similar articles
- Parathyroid-specific deletion of Klotho unravels a novel calcineurin-dependent FGF23 signaling pathway that regulates PTH secretion.
Olauson H, Lindberg K, Amin R, Sato T, Jia T, Goetz R, Mohammadi M, Andersson G, Lanske B, Larsson TE. Olauson H, et al. PLoS Genet. 2013;9(12):e1003975. doi: 10.1371/journal.pgen.1003975. Epub 2013 Dec 12. PLoS Genet. 2013. PMID: 24348262 Free PMC article. - Parathyroid function in chronic kidney disease: role of FGF23-Klotho axis.
Koizumi M, Komaba H, Fukagawa M. Koizumi M, et al. Contrib Nephrol. 2013;180:110-23. doi: 10.1159/000346791. Epub 2013 May 3. Contrib Nephrol. 2013. PMID: 23652554 Review. - Fgf23 and parathyroid hormone signaling interact in kidney and bone.
Andrukhova O, Streicher C, Zeitz U, Erben RG. Andrukhova O, et al. Mol Cell Endocrinol. 2016 Nov 15;436:224-39. doi: 10.1016/j.mce.2016.07.035. Epub 2016 Aug 4. Mol Cell Endocrinol. 2016. PMID: 27498418 - FGF23 fails to inhibit uremic parathyroid glands.
Canalejo R, Canalejo A, Martinez-Moreno JM, Rodriguez-Ortiz ME, Estepa JC, Mendoza FJ, Munoz-Castaneda JR, Shalhoub V, Almaden Y, Rodriguez M. Canalejo R, et al. J Am Soc Nephrol. 2010 Jul;21(7):1125-35. doi: 10.1681/ASN.2009040427. Epub 2010 Apr 29. J Am Soc Nephrol. 2010. PMID: 20431039 Free PMC article. - Bone mineralization is regulated by signaling cross talk between molecular factors of local and systemic origin: the role of fibroblast growth factor 23.
Sapir-Koren R, Livshits G. Sapir-Koren R, et al. Biofactors. 2014 Nov-Dec;40(6):555-68. doi: 10.1002/biof.1186. Epub 2014 Oct 29. Biofactors. 2014. PMID: 25352227 Review.
Cited by
- The consequences of chronic kidney disease on bone metabolism and growth in children.
Bacchetta J, Harambat J, Cochat P, Salusky IB, Wesseling-Perry K. Bacchetta J, et al. Nephrol Dial Transplant. 2012 Aug;27(8):3063-71. doi: 10.1093/ndt/gfs299. Nephrol Dial Transplant. 2012. PMID: 22851629 Free PMC article. Review. - Targeted deletion of Klotho in kidney distal tubule disrupts mineral metabolism.
Olauson H, Lindberg K, Amin R, Jia T, Wernerson A, Andersson G, Larsson TE. Olauson H, et al. J Am Soc Nephrol. 2012 Oct;23(10):1641-51. doi: 10.1681/ASN.2012010048. Epub 2012 Aug 9. J Am Soc Nephrol. 2012. PMID: 22878961 Free PMC article. - Parathyroid diseases and animal models.
Imanishi Y, Nagata Y, Inaba M. Imanishi Y, et al. Front Endocrinol (Lausanne). 2012 Jun 27;3:78. doi: 10.3389/fendo.2012.00078. eCollection 2012. Front Endocrinol (Lausanne). 2012. PMID: 22754549 Free PMC article. - Fibroblast growth factor 23 enhances renal klotho abundance.
Takenaka T, Watanabe Y, Inoue T, Miyazaki T, Suzuki H. Takenaka T, et al. Pflugers Arch. 2013 Jul;465(7):935-43. doi: 10.1007/s00424-013-1226-z. Epub 2013 Mar 7. Pflugers Arch. 2013. PMID: 23467972 - The osteocyte plays multiple roles in bone remodeling and mineral homeostasis.
Chen H, Senda T, Kubo KY. Chen H, et al. Med Mol Morphol. 2015 Jun;48(2):61-8. doi: 10.1007/s00795-015-0099-y. Epub 2015 Mar 20. Med Mol Morphol. 2015. PMID: 25791218 Review.
References
- Liu S., et al. Pathogenic role of Fgf23 in Hyp mice. Am. J. Physiol. Endocrinol. Metab. 2006;291:E38–E49. - PubMed
- Kolek O.I., et al. 1alpha,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289:G1036–G1042. - PubMed
- Liu S., et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J. Am. Soc. Nephrol. 2006;17:1305–1315. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous