Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice - PubMed (original) (raw)
Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice
Laurent Yvan-Charvet et al. J Clin Invest. 2007 Dec.
Abstract
HDLs protect against the development of atherosclerosis, but the underlying mechanisms are poorly understood. HDL and its apolipoproteins can promote cholesterol efflux from macrophage foam cells via the ATP-binding cassette transporters ABCA1 and ABCG1. Experiments addressing the individual roles of ABCA1 and ABCG1 in the development of atherosclerosis have produced mixed results, perhaps because of compensatory upregulation in the individual KO models. To clarify the role of transporter-mediated sterol efflux in this disease process, we transplanted BM from Abca1(-/-)Abcg1(-/-) mice into LDL receptor-deficient mice and administered a high-cholesterol diet. Compared with control and single-KO BM recipients, Abca1(-/-)Abcg1(-/-) BM recipients showed accelerated atherosclerosis and extensive infiltration of the myocardium and spleen with macrophage foam cells. In experiments with isolated macrophages, combined ABCA1 and ABCG1 deficiency resulted in impaired cholesterol efflux to HDL or apoA-1, profoundly decreased apoE secretion, and increased secretion of inflammatory cytokines and chemokines. In addition, these cells showed increased apoptosis when challenged with free cholesterol or oxidized LDL loading. These results suggest that the combined effects of ABCA1 and ABCG1 in mediating macrophage sterol efflux are central to the antiatherogenic properties of HDL.
Figures
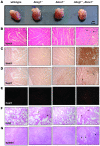
Figure 1. Foam cell infiltration of the myocardium in Abca1–/–Abcg1–/– BM recipients but not WT or single-KO recipients.
(A) Representative hearts obtained from Ldlr+/– mice transplanted with BM derived from WT, Abcg1–/–, Abca1–/–, or Abca1–/–Abcg1–/– mice and fed a high-cholesterol diet for 12 weeks. Hearts from all Abca1–/–Abcg1–/– recipients were visibly smaller and pale compared with WT and single-KO controls. Scale bar: 1 cm. (B–G) Paraffin-embedded serial sections obtained from the myocardium surrounding the proximal aorta, lung, and spleen of mice transplanted with BM of all 4 genotypes. Original magnification, ×200. (B) H&E staining revealed an extensive cellular infiltrate in the myocardium of Abca1–/–Abcg1–/– recipients (9 of 9) but not controls (WT, 0 of 12; Abcg1–/–, 0 of 8; Abca1–/–, 0 of 16). The infiltrate resembled foam cell lesions of the vessel wall, including apparent cholesterol clefts (arrows). (C) Mac-3 immunostaining (dark brown) confirmed that the majority of cells were macrophages. (D) MCA771G immunostaining (dark brown) revealed a moderate number of neutrophils (arrows) as well. (E) TUNEL staining revealed pockets of apoptotic cells (red) in the infiltrated regions of myocardium from Abca1–/–Abcg1–/– recipients (7 of 9) only. (F and G) H&E staining revealed an extensive cellular infiltrate in the lungs and spleens of _Abca1–/–Abcg1–/–_recipients as well as in the lungs of Abcg1–/– recipients. Arrows indicate foam cells.
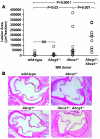
Figure 2. Atherosclerotic lesion development in the proximal aorta.
(A) Quantification of proximal aortic root lesion area by morphometric analysis of H&E-stained sections in Ldlr+/– mice transplanted with WT (n = 12), Abcg1–/– (n = 8), Abca1–/– (n = 16), or Abca1–/–Abcg1–/– (n = 9) BM after 12 weeks of high-cholesterol diet. Values for individual mice are shown as open circles, representing the average of 5 sections per mouse. Horizontal bars indicate the group medians: control, 3,994 μm2/section; Abcg1–/–, 3,724 μm2/section; Abca1–/–, 40,070 μm2/section; Abca1–/–Abcg1–/–, 186,182 μm2/section. Mean lesion areas (mean ± SEM) were as follows: control, 9,090 ± 3,872 μm2/section; Abcg1–/–, 15,412 ± 1,640 μm2/section; Abca1–/–, 72,675 ± 28,879 μm2/section; Abca1–/–Abcg1–/–, 174,958 ± 36,269 μm2/section. ANOVA was performed with square root–transformed data. (B) Representative H&E-stained proximal aortas from mice receiving BM of all the 4 genotypes after 12 weeks of high-cholesterol diet. Original magnification, ×100.
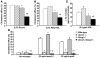
Figure 3. Cholesterol efflux from peritoneal macrophages isolated from WT, Abca1–/–, Abcg1–/–, and Abca1–/–Abcg1–/– mice.
(A, B, and D) Thioglycollate-elicited macrophages were cultured for 24 h in DMEM plus 0.2% BSA containing 50 μg/ml acLDL, 3 μmol/l T0901317, and 2 μCi/ml [3H]-cholesterol. Then, a pool of human serum (2.5%; A), a pool of human serum devoid of apoB-containing particles (2.5%; B), and media devoid of acceptors or containing lipid-poor apoA-1 or apoE (D) were added as acceptor and incubated for 6 h before the media and cells were collected for analysis. (C) Cholesterol efflux was also confirmed by cholesterol mass analysis. Cells were cultured overnight with 50 μg/ml acLDL and 3 μmol/l T0901317 and then incubated for 6 hours with 50 μg/ml human HDL isolated by ultracentrifugation. The HDL-mediated net cholesterol efflux (ΔTC) was calculated as the difference of cholesterol mass of the medium with and without cells. Values are mean ± SEM. *P < 0.05 versus WT. Χ_P_ < 0.05 versus single-KOs.
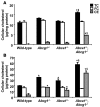
Figure 4. Cellular cholesterol mass in peritoneal macrophages from WT, Abcg1–/–, Abca1–/–, and Abca1–/–Abcg1–/– macrophages.
Thioglycollate-elicited macrophages were harvested from WT, Abcg1–/–, Abca1–/–, and Abca1–/–Abcg1–/– mice fed standard chow (A) or high-cholesterol diet for 2 weeks (B). After a 1-hour incubation at 37°C, nonadherent cells were removed and adherent cells consisting of macrophages were directly used to estimate cellular cholesterol and cholesteryl ester mass content by gas-liquid chromatography. TC, total cholesterol; FC, free cholesterol; CE, cholesteryl esters. Values are mean ± SEM. *P < 0.05 versus WT. Χ_P_ < 0.05 versus single-KOs.
Figure 5. Western blots showing ApoE protein in media and cell lysates of peritoneal macrophages derived from WT, Abcg1–/–, Abca1–/–, and _Abca1–/–Abcg1–/–_mice.
Thioglycollate-elicited peritoneal macrophages were loaded with 50 μg/ml acLDL and treated with LXR activator (3 μmol/l T0901317) for 24 h and then incubated for 16 h in DMEM plus 0.2% BSA. Equivalent volumes of media normalized for cellular protein levels and equal amounts of cellular proteins (20 μg) from each sample were used for western blot analysis. Numbers below blots indicate change in KO cells compared with loaded control cells from 3 independent experiments. *P < 0.05 versus WT. Χ_P_ < 0.05 versus single KOs.
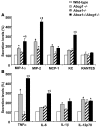
Figure 6. Inflammatory and chemokine gene expression in WT, Abcg1–/–, Abca1–/–, and Abca1–/–Abcg1–/– macrophages.
Peritoneal macrophages were harvested from WT, Abcg1–/–, Abca1–/–, and Abca1–/–Abcg1–/– mice fed high-cholesterol diet for 2 weeks. After a 1-h incubation at 37°C, nonadherent cells were removed and adherent cells consisting of macrophages were incubated in 0.2% BSA DMEM. After 6 h, media were used for secretion analysis. (A) Secretion of chemokines MIP-1α, MIP-2, and MCP-1. KC, growth-regulated oncogene α. (B) Secretion of inflammatory cytokines IL-6, TNF-α, and IL-1β. Secretion levels were normalized to cellular protein amount and expressed as a percentage of WT. Values are mean ± SEM. *P < 0.05 versus WT. Χ_P_ < 0.05 versus single-KOs.
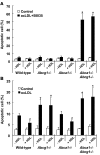
Figure 7. Apoptosis of peritoneal macrophages after loading with free cholesterol loading or oxidized LDL.
Peritoneal macrophages were cultured in DMEM plus 0.2% BSA containing 100 μg/ml acLDL plus ACAT inhibitor (compound 58035; A) or 100 μg/ml Cu-oxidized LDL (oxLDL; B) for 24 h in the presence or absence of 100 μg/ml human HDL. Apoptosis of macrophages was determined by annexin V staining. Results are mean ± SEM. *P < 0.05, genotype effect. #P < 0.05, HDL effect.
Comment in
- J Clin Invest. 117:false.
Similar articles
- Activation of liver X receptor decreases atherosclerosis in Ldlr⁻/⁻ mice in the absence of ATP-binding cassette transporters A1 and G1 in myeloid cells.
Kappus MS, Murphy AJ, Abramowicz S, Ntonga V, Welch CL, Tall AR, Westerterp M. Kappus MS, et al. Arterioscler Thromb Vasc Biol. 2014 Feb;34(2):279-84. doi: 10.1161/ATVBAHA.113.302781. Epub 2013 Dec 5. Arterioscler Thromb Vasc Biol. 2014. PMID: 24311381 Free PMC article. - Deficiency of ATP-binding cassette transporters A1 and G1 in macrophages increases inflammation and accelerates atherosclerosis in mice.
Westerterp M, Murphy AJ, Wang M, Pagler TA, Vengrenyuk Y, Kappus MS, Gorman DJ, Nagareddy PR, Zhu X, Abramowicz S, Parks JS, Welch C, Fisher EA, Wang N, Yvan-Charvet L, Tall AR. Westerterp M, et al. Circ Res. 2013 May 24;112(11):1456-65. doi: 10.1161/CIRCRESAHA.113.301086. Epub 2013 Apr 9. Circ Res. 2013. PMID: 23572498 Free PMC article. - Combined deletion of macrophage ABCA1 and ABCG1 leads to massive lipid accumulation in tissue macrophages and distinct atherosclerosis at relatively low plasma cholesterol levels.
Out R, Hoekstra M, Habets K, Meurs I, de Waard V, Hildebrand RB, Wang Y, Chimini G, Kuiper J, Van Berkel TJ, Van Eck M. Out R, et al. Arterioscler Thromb Vasc Biol. 2008 Feb;28(2):258-64. doi: 10.1161/ATVBAHA.107.156935. Epub 2007 Nov 15. Arterioscler Thromb Vasc Biol. 2008. PMID: 18006857 - Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses.
Yvan-Charvet L, Wang N, Tall AR. Yvan-Charvet L, et al. Arterioscler Thromb Vasc Biol. 2010 Feb;30(2):139-43. doi: 10.1161/ATVBAHA.108.179283. Epub 2009 Oct 1. Arterioscler Thromb Vasc Biol. 2010. PMID: 19797709 Free PMC article. Review. - ATP-binding cassette transporters A1 and G1, HDL metabolism, cholesterol efflux, and inflammation: important targets for the treatment of atherosclerosis.
Ye D, Lammers B, Zhao Y, Meurs I, Van Berkel TJ, Van Eck M. Ye D, et al. Curr Drug Targets. 2011 May;12(5):647-60. doi: 10.2174/138945011795378522. Curr Drug Targets. 2011. PMID: 21039336 Review.
Cited by
- Myeloid cell-specific serine palmitoyltransferase subunit 2 haploinsufficiency reduces murine atherosclerosis.
Chakraborty M, Lou C, Huan C, Kuo MS, Park TS, Cao G, Jiang XC. Chakraborty M, et al. J Clin Invest. 2013 Apr;123(4):1784-97. doi: 10.1172/JCI60415. Epub 2013 Mar 15. J Clin Invest. 2013. PMID: 23549085 Free PMC article. - Impact of Perturbed Pancreatic β-Cell Cholesterol Homeostasis on Adipose Tissue and Skeletal Muscle Metabolism.
Cochran BJ, Hou L, Manavalan AP, Moore BM, Tabet F, Sultana A, Cuesta Torres L, Tang S, Shrestha S, Senanayake P, Patel M, Ryder WJ, Bongers A, Maraninchi M, Wasinger VC, Westerterp M, Tall AR, Barter PJ, Rye KA. Cochran BJ, et al. Diabetes. 2016 Dec;65(12):3610-3620. doi: 10.2337/db16-0668. Epub 2016 Oct 4. Diabetes. 2016. PMID: 27702832 Free PMC article. - Structural analysis identifies an escape route from the adverse lipogenic effects of liver X receptor ligands.
Belorusova AY, Evertsson E, Hovdal D, Sandmark J, Bratt E, Maxvall I, Schulman IG, Åkerblad P, Lindstedt EL. Belorusova AY, et al. Commun Biol. 2019 Nov 22;2:431. doi: 10.1038/s42003-019-0675-0. eCollection 2019. Commun Biol. 2019. PMID: 31799433 Free PMC article. - Cholesterol in beta-cell dysfunction: the emerging connection between HDL cholesterol and type 2 diabetes.
Brunham LR, Kruit JK, Hayden MR, Verchere CB. Brunham LR, et al. Curr Diab Rep. 2010 Feb;10(1):55-60. doi: 10.1007/s11892-009-0090-x. Curr Diab Rep. 2010. PMID: 20425068 Review. - Vulnerable Plaque, Characteristics, Detection, and Potential Therapies.
Hafiane A. Hafiane A. J Cardiovasc Dev Dis. 2019 Jul 27;6(3):26. doi: 10.3390/jcdd6030026. J Cardiovasc Dev Dis. 2019. PMID: 31357630 Free PMC article. Review.
References
- Gordon D.J., Rifkind B.M. High-density lipoprotein-the clinical implications of recent studies. N. Engl. J. Med. 1989;321:1311–1316. - PubMed
- Glomset J.A. The plasma lecithins:cholesterol acyltransferase reaction. J. Lipid Res. 1968;9:155–167. - PubMed
- Kontush A., Chapman M.J. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol. Rev. 2006;58:342–374. - PubMed
- Ross R. Atherosclerosis — an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous